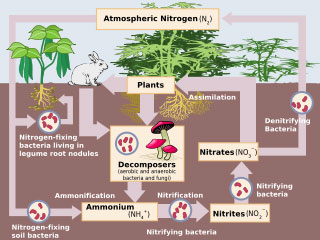
 In the same way that living things require oxygen and CO2 in order to survive, they also need nitrogen (N2) to grow. Nitrogen is present at a level of roughly 15% in the structure of the nucleic acids, proteins and vitamins in the body. It represents one of the ba sic building blocks of life. Around 78% of the atmosphere consists of nitrogen gas, but living things cannot absorb this nitrogen in the air, despite their need of it. It must somehow be turned into a form that living things can use, and then be recycled into the atmosphere so that it does not run out.
In the same way that living things require oxygen and CO2 in order to survive, they also need nitrogen (N2) to grow. Nitrogen is present at a level of roughly 15% in the structure of the nucleic acids, proteins and vitamins in the body. It represents one of the ba sic building blocks of life. Around 78% of the atmosphere consists of nitrogen gas, but living things cannot absorb this nitrogen in the air, despite their need of it. It must somehow be turned into a form that living things can use, and then be recycled into the atmosphere so that it does not run out.
This need too is met by microscopic bacteria.
Plants need to absorb nitrogen from the atmosphere, since they are unable to use it in gas form. Nitrogen is transformed into nitrite by bacteria, and nitrite into nitrates by different bacteria, thus making it capable of being used by plants. But how does this cycle begin?
Nitrogen reaches the Earth in various forms. Atmospheric nitrogen returns to Earth in the form of nitric acid in rain, as the result of phenomena such as lightning. Nitric acid is turned into nitrates by bacteria in the soil, and plants are able to absorb it in that form.
Another cycle is the direct absorption of nitrogen from the air into the soil. Bacteria in the roots of certain plants such as peas and beans, and other legumes take the nitrogen in the air into the soil. At this stage, we encounter a most superior Creation. Proteins, nucleic acid and the majority of organelles all need nitrogen, the most important element in the development of living organisms.. One of the world's most beneficial partnerships exists between plants, which need nitrogen in order to grow, and the bacteria that meet that need. Plants' roots give off special nutrients to attract bacteria. Later, the bacteria enter through special gaps that open in the roots, settle there, and establish nodules by multiplying to enormous levels.
We are indebted to the nitrogen cycle, essential for the maintenance of ecological equilibrium, for the greater part of the vegetables, plants and cereals we eat. As bacteria, described as "simple" by evolutionists, implement the nitrogen cycle, they work like living chemistry factories, and ever since the day they were first created have been performing chemical reactions that may not mean very much to those not closely involved with chemistry. The resolution of the nitrogen fixing reaction summarized below in chemical terms represented a major success for scientists:
N2 + 8H+ + 8e- + 16 ATP = 2NH3 + H2 + 16ADP + 16 Pi
But for this reaction to take place, there needs to be a second support reaction like photosynthesis, respiration or fermentation. These formulae, so baffling to most people, are ordinary everyday work for bacteria. Of course they have undergone no specialized chemical training in order to carry out these chemical processes. Every new bacterium that enters the world is equipped with knowledge and materials that could only belong to a specially trained chemist. In addition, these processes are not limited to bacteria in plant roots. Despite being found in very different places and having very different structures, nitrobacteria, beijerinckia, klebsiella, cyanobacteria, clostridium, desulfovibrio, purple sulphur bacteria, non-purple sulphur bacteria, green sulphur bacteria, rhizobium frankia, azospirillum and a great many more carry out the same reaction, with the same data and programming, in a prefect manner. Furthermore, with the different systems and reactions inside them, these bacteria exhibit structures that are not simple at all.
For example, the nitrogenase enzyme complex that bacteria use during this process is exceptionally sensitive to oxygen. When deprived of oxygen, it stops its activity, for which reason proteins enter into reactions with iron compounds. This represents no problem for anaerobic bacteria, which are capable of living without oxygen, but a major hurdle for bacteria such as cyanobacteria that produce oxygen by photosynthesis. and azotobacter that live freely in the soil. However, these other bacteria have been equipped with various mechanisms to resolve this difficulty. For example, azotobacter species possess metabolisms with the highest known respiratory rate among all organisms, thus keeping oxygen levels in the cells low and protecting the enzyme. In addition, azotobacter species produce high levels of extracellular polysaccharide, compounds consisting of multiple sugars and especially starch used in the formation of the cell wall. Bacteria preserve water in the sticky fluid formed by these compounds and restrict the level at which oxygen is disseminated in the cell. Bacteria like Rhizobium that fix nitrogen in plant roots, possess molecules such as leghemoglobin that consume oxygen. Leghemoglobin serves the same purpose as hemoglobin in animals, regulating oxygen for the node tissues. Interestingly, leghemoglobin is found only in the root nodes and produced only after a plant-bacterium relationship is established. Bacteria that live alone or plants that live without bacteria are unable to manufacture it.
The enzyme nitrogenase, responsible of preserving the nitrogen cycle, breaks down when deprived of oxygen. In that case, the systems that prevent oxygen from reaching the enzyme, and the organisms that produce them must have come into being at the same time as that enzyme. Otherwise, the moment that the enzyme nitrogenase formed, oxygen would have broken it down. The theory of evolution is unable to admit this, because it holds that organisms can form only through gradual mutations. Again according to that theory, either the enzyme nitrogenase or the systems that consume oxygen must have appeared first—yet that illogical sequence that permits no system at all to form. No system can control oxygen in the absence of the enzyme nitrogenase.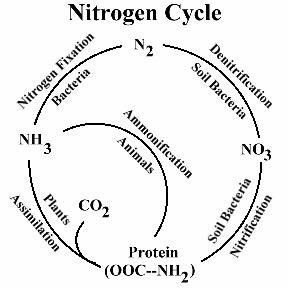 When these bacteria die and are broken down, ammonia is released. At the same time, saprophyte bacteria break down proteins in animal and plant remains and turn them into ammonia. The ammonia, formed in the soil in this way, is converted in the same way into nitrite by nitrite bacteria, and then into nitrate by nitrate bacteria. By this process, known as nitrification, the nitrogen cycle is completed. Nitrate is a form of nitrogen that plants can absorb. This nitrate also reaches human beings and the animals that consume plants for food. By these means, therefore, the needs of all living things are met.
When these bacteria die and are broken down, ammonia is released. At the same time, saprophyte bacteria break down proteins in animal and plant remains and turn them into ammonia. The ammonia, formed in the soil in this way, is converted in the same way into nitrite by nitrite bacteria, and then into nitrate by nitrate bacteria. By this process, known as nitrification, the nitrogen cycle is completed. Nitrate is a form of nitrogen that plants can absorb. This nitrate also reaches human beings and the animals that consume plants for food. By these means, therefore, the needs of all living things are met.
Bacteria have possessed secrets that have guaranteed life on Earth for billions of years. The reason is that they are the flawless work of Allah, with His superior intellect. Allah displays His astonishing artistry in such a magnificent ways so that human beings may witness it, and reflect on what they see.
TOPICS
VaticanSocialismIlluminationFrench RevolutionConvertSabbateanJacobinismMasonic MediaPolitical ZionismYoung TurkThe Committee of Union and ProgressAbdulhamidAnti-NaziWorld Zionist Organization Nuremberg LawsMussoliniWorld War IAdolf EichmannGoyimThe Rothschild DynastyThink-ThankCFRRockefellerCold WarStalinOctober RevolutionSoviet UnionBilderbergVietnamAIPACLobbyfairSoutheastGreeceNew World OrderRed Seageopoliticsveterantaxcustoms2023antelopebullEurasia Islam CouncilNobel PrizeHospitalSocial Security InstitutionAli BabacanTurgut OzalassassinationGaffar OkkanMuhsin YazıcıoğluRosetta NebulaAstronomyRose


