One of the theory of evolution's greatest errors is maintaining that the complex structure of life, with such superior characteristics and processes, came into existence spontaneously, by chance. Back in the 19th century when Charles Darwin first proposed his theory, very little was known about the basic structure of life. Under the microscopes of the day, the cell resembled nothing more than a blot, which some described it as "a jelly-like substance." For that reason, when Darwin claimed that life arose through the spontaneous and chance development of a cell, he received little opposition. However, later science and technology (during the second half of the 20th century in particular) revealed just what a complex and superior structure the cell actually possessed, together with a great many features that could not have come into being by chance, as Darwinists maintained. Instead, the cell rather resembled a biochemical factory, but one superior to any on Earth.
 |
As has been discussed throughout this book, proteins and other cellular subcomponents all possess exceedingly complex structures, and among them is an extraordinary organization and impeccable planning. Every protein fulfils vitally important functions in the human body; with a plan so detailed as to amaze. It is utterly illogical to maintain that such structures emerged after inanimate and unconscious atoms came together by chance to form such complex structures, with flawless organization and a division of labor. Yet Darwinists still blindly defend the theory of evolution, despite its having been discredited scientifically, solely in order to keep alive their materialist ideologies and deny the existence of a Creator. They shamelessly set out their most irrational claims, even using false proofs to influence uninformed people who do seldom reflect on these issues.
For example, in order to make the theory sound convincing, a Turkish evolutionist wishing to propound the theory of evolution describes the chance appearance of proteins as something very easy. Yet someone with only the most basic knowledge of proteins can recognize the bias and distortions in his account:
Evolution is the passage, in both animate and inanimate nature, from the simple to the complex, over the course of time (over billions of years; through millions or even billions of reactions). To formularize, the process began with two elements for example; let us say that the odds of A combining with B are fifty percent. Once AB has formed, the odds of C joining are also fifty percent. The odds of D then combining with ABC are fifty percent, or similar probabilities. The idea that this happened in a moment, and the impossibility of this, cannot be laid at Darwinists' door. 49
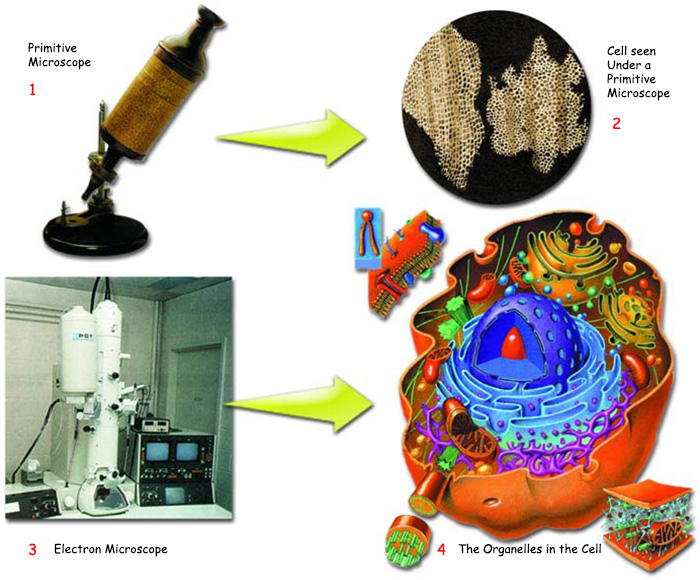 | |
| 1. Primitive microscope | 3. Electron microscope |
| As shown above, any cell viewed under the primitive microscopes used in the 19th century appeared to be a mere blot. | |
These words describe a scenario astonishing to anyone with the slightest knowledge of biochemistry. This evolutionist is unaware or else ignoring the facts that proteins consist of strings of amino acids arranged as if on a bead necklace; that there are 20 different types of amino acids; and that even more importantly, for a chain of amino acids to be regarded as a protein, they must be arranged in a specific order.
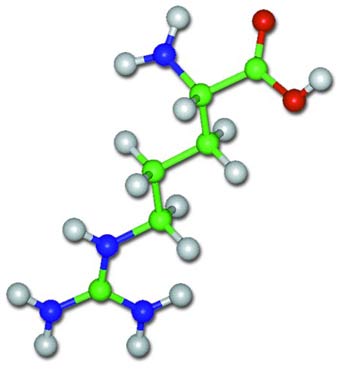 |
| An amino acid chain |
This is like imagining that a poem is a random combination of letters and then saying, "It's easy for a poem to emerge by chance. Put two letters together, then a third and then a fourth, and you can easily wind up with a poem thousands of letters long." In fact, however, in order for a poem to emerge, letters need to be set out in a particular sequence to acquire meaning. And amino acids are arranged to constitute proteins in a far more difficult and complex process.
Since amino acid strings must be arranged in a particular order to produce a protein, the odds of such a sequence coming about by chance are zero. (For instance, the odds of 400 amino acids adopting a specific sequence are 1 in 10520 —in other words, the chances are 1 followed by 520 zeros.)
Even the most dyed-in-the-wool Darwinists accept the fact that proteins cannot emerge by chance. As one example, the Russian scientist Alexander Oparin, regarded as the father of the theory of molecular evolution, said: "The spontaneous formation of such an atomic arrangement in the protein molecule would seem as improbable as the accidental origin of Virgil's Aeneid from scattered letters." 50
The same calculations have been performed, and the same probability figures obtained, by such well-known Darwinists as David Shapiro, Harold Morovitz, Francis Crick, Carl Sagan, Lecompte du Nuoy and Frank Salisbury.
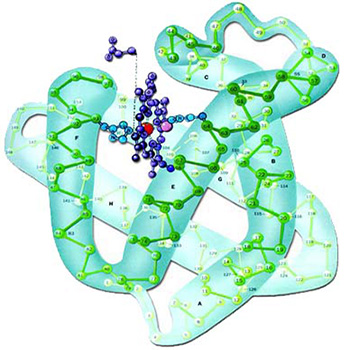
For years, it has been known that every protein's properties and functions depend on its amino acid sequence and bonds. For example, the protein histone turns into a three-dimensional shape with a positive charge distributed on its surface. As a result of this shape and charge distribution, it enables DNA to adopt an appropriate form and to store data. The density of data storage in DNA is thus several billion times that of the most advanced computers.51 And by means of this protein, the DNA molecules possess the capacity to store and encode all the information in the body.
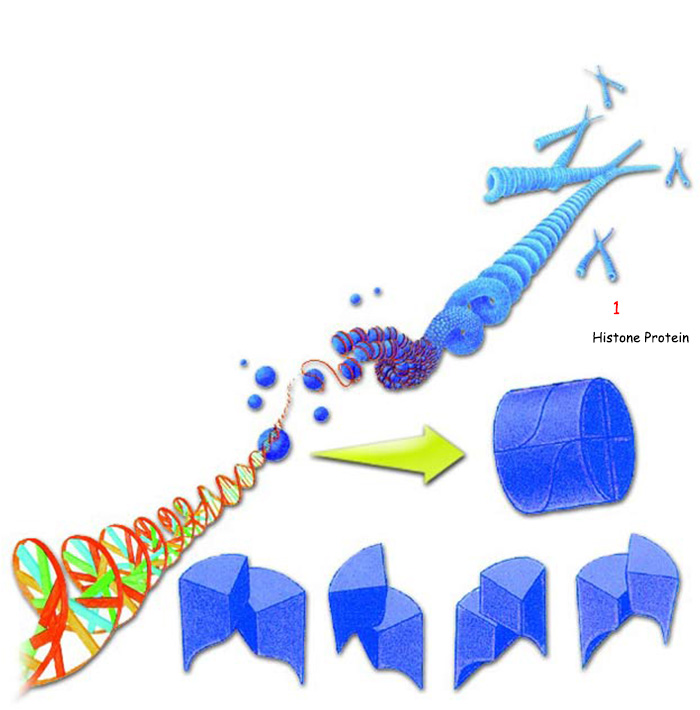 |
| 1. Histone Protein |
| Due to its structure, the protein histone assumes a three-dimensional form that lets DNA revolve around itself and store information. |
With the discovery that proteins and DNA molecules have such a complex structure, it was understood that even were the whole universe filled with amino acids, still life could never emerge from them spontaneously. The evolutionist geologist William Stokes admits this fact:
[Protein] would not occur during billions of years on billions of planets, each covered by a blanket of a concentrated watery solution of the necessary amino acids. 52
In addition, as stated before, a number of preconditions must be met before even a single protein molecule can form, making this definitely impossible. To briefly summarize some of them:
- For even the smallest protein to form, hundreds of amino acids have to be arranged in specific numbers, varieties and sequences.
- A single amino acid too many—or too few, or in the wrong place—will render the protein useless.
 |
| All the amino acids in a protein chain must be left-handed. If just one is right-handed, then the protein chain becomes non-functional. |
- All the amino acids in a protein need to be left-handed. The appearance of a single right-handed amino acid will impair the protein's structure.
- The protein's three-dimensional structure endows it with functionality. Protein synthesis is carried out in the ribosome inside the cell with the help of special enzymes, and in a wide variety of proteins, this three-dimensional form cannot form spontaneously. Therefore, when the first functional protein came into being, other enzymes must have existed beforehand—which demonstrates the invalidity of the theory of evolution.
- Proteins cannot be synthesized without enzymes, and enzymes are all proteins.
- Around 100 proteins need to be present in order for a single protein to be synthesized. There therefore need to be proteins for proteins to exist.
- DNA manufactures the protein-synthesizing enzymes. Protein cannot be synthesized without DNA. DNA is therefore also needed in order for proteins to form.
- All the organelles in the cell have important tasks in protein synthesis. In other words, in order for proteins to form a perfect and fully functioning cell needs to exist together with all its organelles.
It is definitely impossible for even one of these preconditions to have come into existence by chance. Other proteins need to be in existence for a protein to form and this totally eliminates the possibility of a protein forming by chance.
Another point that Darwinists hope to ignore is that in order for life to emerge, all the necessary components must be present together at the same time. All these components need to be fully formed if they are to serve any purpose. A flawed structure cannot function and—according to the theory of evolution's own claims— will be eliminated under natural conditions. This irreducible complexity represents one of those factors that demolish the theory of evolution.
The prominent Turkish evolutionist Professor Ali Demirsoy describes how all their components must be present together in order for living structures to become functional:
The most crucial point of the problem is how mitochondria acquired this property. For a single individual to acquire this feature as the result of chance, we have to combine an inconceivable number of such infinitesimal possibilities... Enzymes, which permit respiration and serve as catalysts in different forms at every level, represent the essence of the mechanism. A cell either possesses this full string of enzymes, or else it is meaningless. If some enzymes are missing, no result can emerge. In order not to conflict with scientific thinking and not to engage in a more dogmatic explanation and speculation we must accept, albeit unwillingly, that all the respiratory enzymes are present in the cell at one time and with none missing, before making contact with oxygen. 53
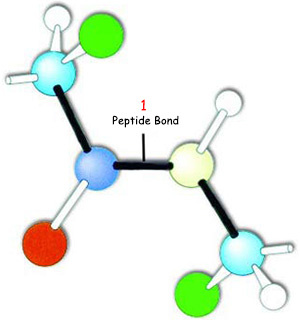 |
| Peptide bond |
| The bonds attaching the amino acids that comprise proteins must all be peptide bonds. |
In a despairing tone, this evolutionist states that all the respiratory enzymes must be present at the same time in the cell. This means that all the organs, cells, enzymes and mechanisms of the respiratory system must have been created at one and the same time. Yet for some reason, this scientist views this self-evident truth as dogmatic and speculative, contrary to scientific thinking, and avoids admitting the facts. Yet in reality, denying the proofs of creation that are plain to see represents a dogmatic violation of scientific thinking.
Professor Russell Doolittle, another world-famous evolutionist, admits that the very existence of proteins and their ability to function depend on other proteins—and that this represents an impasse for Darwinists:
How in the world did this complex and delicately balanced process evolve?…The paradox was, if each protein depended on activation by another, how could the system ever have arisen? Of what use would any part of the scheme be without the whole ensemble? 54
In the present day, a great many Darwinists honestly confess the impossibility of proteins and life emerging by chance. However, they still continue to defend the theory for the sake of their ideologies. Below you'll find a number of statements by world-famous Darwinists admitting the impossibility of proteins coming into existence as a result of coincidence:
Harold Blum: "The spontaneous formation of a polypeptide of the size of the smallest known proteins seems beyond all probability." 55
Hoimar von Ditfurth: "These two polymers [egg white and nucleic acids] have been constructed in such a complex manner and, as if that were not enough, their structures exhibit such a high level of individuality that to imagine these came to that level by acquiring wealth solely as the result of chance goes far beyond being even an astronomically and inconceivably small possibility."56
"The statistical impossibility of the living structures in question emerging as the result of chance alone is a rather current example of the present-day level of development of science. Indeed, looking at those extraordinary individual features in the formations of a single protein carrying out biological functions, it appears impossible to explain a large number of atoms combining together, all in the correct and requisite sequence, at the right time and moment and with the right electrical and mechanical features, all in terms of chance. " 57
"No matter how large the universe may be, chance giving rise to the birth of protein and nucleic acid is [an] impossibility..." 58
David A. Kaufman (Florida University): "Evolution lacks a scientifically acceptable explanation of the source of the precisely planned codes within cells, without which there can be no specific proteins and hence, no life. "59
 |
| Prof.Russel Doolittle |
The information provided throughout this book regarding the structures, functions and production of proteins invisible to the naked eye shows that it is impossible for them to have formed by chance. Remembered that this information about proteins is just a short summary of the total. In addition, there still remain many secrets about proteins that science has yet to fathom.
It's very important that people learn about proteins and other miracles of creation in order to grasp the logical mindset and thinking of those who maintain that proteins came into being by chance. Lacking a good knowledge of the structures of proteins, the cell and enzymes, someone may well attach little importance to a theory that claims they came to be by chance. However, after comprehending the details, that person will understand the serious threat posed by any theory that ascribes divine status to coincidences, and that it needs to be forestalled right away.
Believing in chance despite so much evidence to the contrary signifies a collapse of logic, understanding and comprehension. These people may be professors or researchers who have written dozens of scientific books and may even have won a Nobel Prize, but that does not change the facts.
The collapse of reason by some people refusing to understand what they see and hear is one of the greatest dangers facing humanity. For that reason, those of reason and conscience must prevent that collapse by taking the requisite precautions, and ensure that others receive accurate information and explanatory proofs.
The second reason for learning about proofs of creation such as proteins is the revelation of Allah's infinite might, intellect, knowledge and incomparable creation, and to introduce them to its extraordinary splendor. Those who believe in the existence of Allah reflect on the proofs of His creation on Earth and in the heavens. This enhances their love of Allah, and also their fear of Him. As He has revealed in one verse:
And humanity and beasts and livestock are likewise of varying colors.
Only those of His servants with knowledge have fear of Allah.
Allah is Almighty, Ever-Forgiving.
(Surah Fatir: 28)
 |
| Alexander I.Oparin |
In the 20th century, Darwinists began seeking an answer to the question of how the first cell came into being. The first work on this subject was done by Alexander L. Oparin, a Russian biologist who proposed the "chemical evolution" model. But Oparin was unable to obtain any results from his research, and finally admitted:
Unfortunately, however, the problem of the origin of the cell is perhaps the most obscure point in the whole study of the evolution of organisms. 60
After Oparin, a great many Darwinists performed countless experiments attempting to prove that cells came into being as the result of coincidences, but every one ended in failure. The most highly highly regarded of these doomed experiments was carried out in 1953 by the American researcher Stanley Miller.
Miller prepared a mechanism conforming to Oparin's chemical evolution model. A mixture of the gasses assumed to represent the primordial atmosphere, methane (CH4), ammoniac (NH3), steam (H2O) and hydrogen (H2) was placed in a tank containing an electrical apparatus. Miller then sent a high-voltage electrical charge through the tank to simulate the effect of ultraviolet light on the pre-life atmospheric gasses. He then heated this gas mixture to 100 degrees for a week, while continuing to supply an electrical current, and eventually observed that three of the 20 amino acids essential to life had been synthesized. He immediately separated these molecules from the tank using a mechanism known as the cold trap. Other experiments also obtained various other amino acids under similar conditions.
 |
| Standley Miller |
This experiment carried out by Miller under allegedly primordial conditions was a source of great rejoicing among Darwinists, who portrayed the experiment as an enormous success. From their point of view, the experiment showed that biological building blocks could have been produced from simple atmospheric gasses in the primitive world—an important step in Oparin's scenario, which would thus provide experimental support for Oparin's theory of chemical evolution. Some circles, aware of the experiment's importance, sought to provide their own support for it. The famous astronomer Carl Sagan, for instance, described the Miller-Urey experiment "as the single most significant step in convincing many scientists that life is likely to be abundant in the cosmos."61
Considerable space began to be devoted to the Miller experiment in textbooks and public media such as Time magazine. Inspired by the Miller experiment, fictitious evolutionary scenarios based on chemical evolution—and describing it as the origin of life—lost no time in appearing in school books. Indeed, as a result of this experiment, the belief known as "neovitalism"—the idea that matter possesses the inherent ability to reproduce itself—was resurrected.62
However, the Miller experiment was based on preconceptions of Oparin's and actually contained a great many elements far removed from scientific fact. The experiment was prepared to confirm the theory of chemical evolution that Oparin had dreamed up, and was intended to prove the validity of the theory of evolution. The setup used to produce amino acids bore no relation to the actual atmospheric conditions on the primordial Earth. Furthermore, it included several multilateral mechanisms for the production of amino acids that were not to be found in any natural environment. In the light of scientific standards, this experiment clearly contained prejudiced mechanisms.
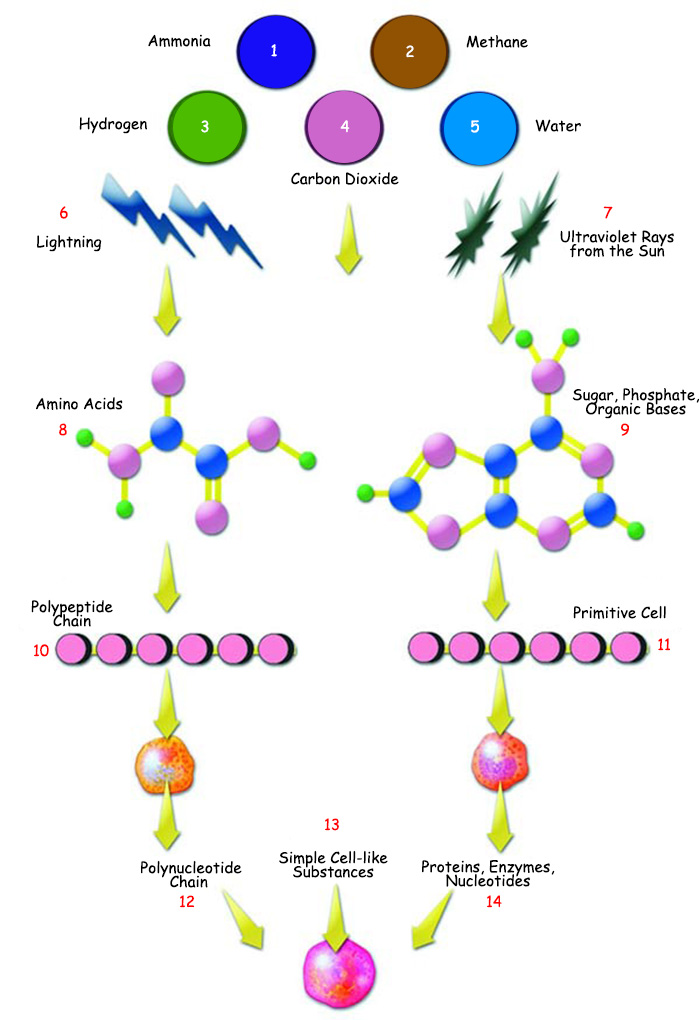 | ||
| 1. Ammonia | 6. Lightning | 11. Primitive Cell |
| Evolutionists maintain that under the conditions on the primordial Earth, unconscious atoms turned into flawless protein molecules by following the progression shown in the diagram. Yet 20th-century science revealed the illogicality of that claim. | ||
Shortly after Miller carried out his experiment formulated to prove that amino acids could form spontaneously under primordial world conditions, it was realized that this experiment was incompatible with the scientific facts in a number of ways. Considering the points that demonstrate the scientific invalidity of the experiment, it is clear that scientific objectivity was not its aim.
When Oparin proposed his theory of chemical evolution, he suggested that the atmosphere on the primordial Earth was very different from what it is today.63 Stanley Miller sought to recreate these primordial atmospheric assumptions set out in Oparin's book in 1936. Therefore, in seeking to reproduce the formation of amino acids, Miller assumed that the primordial atmosphere consisted of methane (CH4), ammonia (NH3) and hydrogen (H2), as hypothesized by Oparin. In addition, he suggested that the Earth's atmosphere did not contain free oxygen. But in the years that followed his experiment, new geochemical evidence and experiments performed in the light of them clearly revealed that Oparin and Miller's estimates were inaccurate. On the contrary, the evidence demonstrated that the dominant gasses in the primordial atmosphere were carbon dioxide, nitrogen and water vapor; there was no methane, ammonia or hydrogen. This information showed that Miller's and similar experiments had been built on a false assumptions.
 |
| Darwinists seek to prove that under the conditions on the primordial Earth, inanimate substances gave rise to proteins by chance. But today, we know that proteins cannot form by chance. |
In any case, however, Miller had used these gasses deliberately. His objective was to prove experimentally the chemical evolution scenario proposed by Oparin 1924. For that reason, in determining the parameters of his experiment, Miller set out to duplicate the conditions Oparin had assumed. In fact, his aim was not to create the authentic primordial atmosphere before the emergence of life, but to produce the requisite atmosphere for amino acids to emerge.
Richard Kerr of Science magazine states that none of the geological and geochemical evidence collected over the last 30 years supports Miller's primordial atmosphere conditions.64 The only reason for continuing to regard the primordial atmosphere conditions as accurate was that the theory of chemical evolution needed this assumption. The primordial atmospheric conditions that Oparin and Miller assumed were the most appropriate ones to allow amino acids to emerge. Under normal conditions in a natural atmosphere, no chemical reactions will take place among atmospheric gasses. Even if they do take place they are not at the level that can give rise to biological building blocks.
Trying to form biological building blocks in a neutral atmosphere is like expecting two inanimate chemicals to react.
In fact, the primordial conditions "recreated" in Miller's experiment and others like it constitute no scientific evidence regarding the origin of life, since they did not take place in the actual primordial environment. After independent geochemical studies demonstrated that in the early atmosphere, chemical conditions prevailed that would never permit amino acids to form, it was realized that Miller's experiment was actually meaningless. Not only do experiments of this kind show that chemical evolution is impossible, but they also prove the presence of a rational Creator in the planning of living systems.
A series of geological studies showed that even prior to the emergence of plant life, significant levels of free oxygen and volcanic gasses were present due to photo dissociation in water evaporation. In rocks estimated to be around 3.5 billion years old, the presence of oxidized iron and uranium showed that there had been oxygen in the atmosphere.65 These findings indicated that the level of oxygen at that time had not been low, as Darwinists maintained, but was actually much higher than they had suggested. Research also showed that during that period, 10,000 times more ultraviolet light reached the Earth than Darwinists had estimated. Inevitably, such intense ultraviolet light would break down the water molecules in the atmosphere, producing free oxygen.
This fact, which Miller neglected to take it into account, made his experiment totally invalid. If he had used oxygen in the experiment, the methane would have broken down into carbon dioxide and water, and ammonia into nitrogen and water. On the other hand, in an oxygen-free atmosphere before the ozone layer had come into existence, any amino acids directly exposed to ultraviolet rays would have broken down. Whether it contained oxygen or not, an atmosphere on the primordial earth would destroy amino acids.
Assuming that Stanley Miller did use gasses that actually resembled those in the primordial atmosphere, shouldn't the results of the experiment support chemical evolution? No! In addition to such building blocks as amino acids and nucleic acid bases, his experiments also produced non-biological substances. Barring human intervention, these substances would enter into reactions with other useful substances, to form chemical compounds with no biological significance. As soon as the amino acids appeared, Miller was obliged to protect them from other substances and from the harmful effects of other conditions in that environment, so his experiment used a mechanism known as the cold trap". Otherwise, the same conditions that gave rise to the amino acids would have destroyed these molecules as soon as they formed.
On the primordial Earth of course, there was no such thing as a cold trap. Yet without one, even if a variety of amino acid were produced, those molecules would immediately be broken down in the prevailing environment. As the chemist Richard Bliss stated, "Actually, without this trap, the chemical products would have been destroyed by the energy source."66
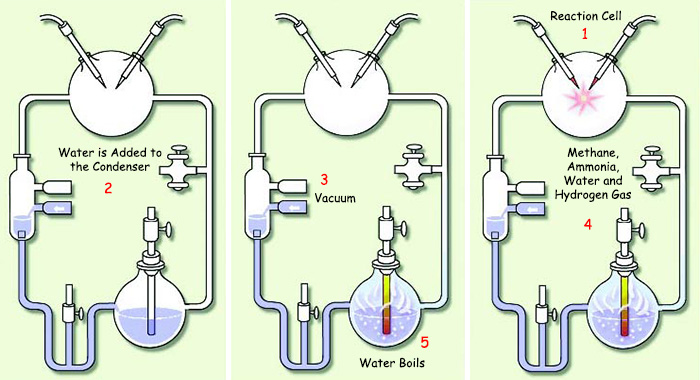 | |
| 1. Reaction Cell | 4. Methane, Ammonia, Water and Hydrogen Gas |
| Stanley Miller's experimental setup. In his experiment, Miller setup a number of conditions that were incompatible with the originals. For that reason, the scientific world has regarded his experiment as invalid. | |
Indeed, before Miller installed a cold trap, he had been unable to obtain a single amino acid in experiments he had performed.
In fact, Miller's experiment totally discredited the claim that life emerged as the result of unconscious coincidences. He demonstrated that amino acids can be obtained only when there is conscious intervention, in a laboratory environment where all the necessary conditions are provided.
Even though the Miller experiment is still depicted as an important scientific discovery in some quarters, it has effectively been abandoned by evolutionary authorities. In recent years, Western scientific journals have stated that in terms of accounting for the origins of life, the experiment is meaningless. For instance, the following comment, appeared in the February 1998 issue of the well-known evolutionist journal Earth, under the title "Life's Crucible":
Geologists now think that the primordial atmosphere consisted mainly of carbon dioxide and nitrogen, gases that are less reactive than those used in the 1953 experiment. And even if Miller's atmosphere could have existed, how do you get simple molecules such as amino acids to go through the necessary chemical changes that will convert them into more complicated compounds, or polymers, such as proteins? Miller himself throws up his hands at that part of the puzzle. "It's a problem," he sighs with exasperation. "How do you make polymers? That's not so easy."67
As you have seen, Miller himself realized that his experiment added nothing to explain the origin of life. In the March 1998 edition of National Geographic magazine, an article titled "The Rise of Life on Earth," contained the following:
Many scientists now suspect that the early atmosphere was different from what Miller first supposed. They think it consisted of carbon dioxide and nitrogen rather than hydrogen, methane, and ammonia. That's bad news for chemists. When they try sparking carbon dioxide and nitrogen, they get a paltry amount of organic molecules—the equivalent of dissolving a drop of food coloring in a swimming pool of water. Scientists find it hard to imagine life emerging from such a diluted soup.68
In short, neither the Miller experiment nor any other evolutionist endeavor can answer the question of how life on Earth appeared. All their researches show the impossibility of life coming into being by chance and therefore, that it was created. Darwinists refuse to accept this because they hold to a series of preconceptions that fly in the face of science. In fact, Harold Urey—Stanley Miller's student and who helped set up his experiment—made the following admission:
All of us who study the origin of life find that the more we look into it, the more we feel it is too complex to have evolved anywhere. We all believe as an article of faith that life evolved from dead matter on this planet. It is just that its complexity is so great, it is hard for us to imagine that it did.69
Despite its invalidity, some Darwinists still seek to use the Miller experiment as proof that amino acids might have formed from inanimate substances. But even if that were the case, it would still not resolve Darwinists' difficulties! Even more impossible hurdles stand in their way: Amino acids would have to combine to form proteins—which are vastly more complex structures. And it is even more unrealistic to maintain that proteins formed by chance under natural conditions. You have already seen the mathematical calculations demonstrating the impossibility of amino acids combining in the sequences needed to give rise to proteins. And it's also chemically impossible for proteins to have emerged in the primordial Earth's atmosphere.
 |
| Sydney Fox |
As already made clear, when amino acids combine to form proteins, they establish special peptide bonds among themselves. When this bond is established, a water molecule is released.
This invalidates the Darwinist account of primordial life emerging in the seas because according to the law of chemistry known as the Le Chatelier principle, any reaction that releases water (a so-called condensation reaction) cannot take place in an environment that contains water. Specifically, such a reaction taking place in a watery environment is described as having the "lowest probability of taking place."
Therefore, the ocean—which Darwinists describe as the place where amino acids formed and life began—is an absolutely unsuitable environment for amino acids to combine and give rise to proteins. 70
But in the face of this fact, proponents of Darwinism cannot alter their claims and maintain that primordial life first appeared on land. The oceans and seas are the only environment that could have protected amino acids from the harmful effects of sunlight coming through the primordial atmosphere. On land, amino acids are quickly broken down by ultraviolet rays. Yet the Le Chatelier principle makes it impossible for them to emerge in the sea. As far as the theory of evolution is concerned, this represents two dead ends.
Faced with the dilemma described above, Darwinist researchers set about producing various scenarios to overcome the "water problem" that demolished all their theories. To resolve the difficulty, the well-known Sydney Fox advanced one interesting theory: that after the first amino acids had formed in the primordial ocean, they must have immediately been cast up onto cliffs near a volcano. The high temperatures on those rocks must have evaporated the water containing amino acids. In this way, the "dried" amino acids could have combined to form proteins.
However, this sophistry convinced no one, because amino acids could not have exhibited heat resistance to the extent proposed by Fox. Research has revealed that at high temperatures, amino acids are immediately destroyed.
 |
| Fox suggested that after amino acids had formed in the ocean, they were washed onto rocks on the side of a volcano. However, since amino acids are unable to withstand such high temperatures, Fox's claim enjoyed little support from scientific circles. |
However, Fox did not give in. Under "very special conditions in the laboratory," he combined purified amino acids by heating them in a dry environment. The amino acids did combine, but Fox still failed to obtain proteins, only randomly connected, simple and irregular amino acid links—a far cry from the proteins in any living thing. In any case, had Fox maintained the amino acids at that same temperature, then the useless links that did emerge would have broken down.71
Another point depriving the experiment of any significance is that rather than the amino acids obtained in the Miller experiment, Fox used the pure amino acids found in living organisms. Since he claimed his experiment to be a continuation of Miller's, he should have started off from where Miller left off. Yet neither Fox nor any other researcher used the useless amino acids that Miller produced.72
Fox's experiment received no welcome from Darwinists because it was plain that the meaningless chains of amino acids (or proteinoids) that Fox obtained could never have emerged under natural conditions. In addition, he had still not obtained the proteins that constitute the building blocks of living things, so the problem of the origin of proteins had still not been resolved. An article published at the time in Chemical Engineering magazine commented on Fox's experiment:
Sydney Fox and the other researchers managed to unite the amino acids in the shape of "proteinoids" by using very special heating techniques under conditions which in fact did not exist at all in the primordial stages of Earth. Also, they are not at all similar to the very regular proteins present in living things. They are nothing but useless, irregular chemical stains. It was explained that even if such molecules had formed in the early ages, they would definitely be destroyed. 73
Indeed, the proteinoids that Fox obtained were far from having the function and structure of real proteins. The difference was as great as between a pile of scrap metal and a complex technological device.
Furthermore, this meaningless collection of amino acids had no way of survival in the primordial atmosphere. Under the destructive conditions of the time, intense ultraviolet rays and uncontrolled natural phenomena would cause these proteinoids to break down with no opportunity to continue combining. The Le Chatelier principle removes any question of amino acids being underwater where ultraviolet rays could not reach them. In light of these facts, scientists rapidly began to doubt the hypothesis that proteinoid molecules could represent the beginning of life.
49.Alaeddin Senel, "The Evolution Deception? Or the Deception of the Age?," Bilim ve Utopya Magazine, December 1998. 
50.Alexander I. Oparin, Origin of Life, New York: Dover Publications, 1936 (Reprint,1953), pp. 132-133. 
51.Stephen C. Meyer, The Intercollegiate Review 31, No: 2 (Spring 1996). 
52.W. R. Bird, The Origin of Species Revisited, Nashville: Thomas Nelson Co., 1991, p. 305. 
53.Ali Demirsoy, Kalitim ve Evrim ("Inheritance and Evolution"), Ankara: Meteksan Publishing Co., 1984, p. 94. 
54.Michael Behe, Darwin's Black Box, p. 91; Prof. Russell Doolittle, "The Comparative Biochemistry of Blood Coagulation", Harvard PhD thesis, 1961. 
55.W. R. Bird, The Origin of Species Revisited, Nashville: Thomas Nelson Co., 1991, p. 304. 
56.Hoimar Von Ditfurth, Dinozorlarin Sessiz Gecesi 1, ["The Silent Night of the Dinosaurs1"], p. 122. 
59.David B. Loughran, SBS Vital Topics, April 1996, Stewarton Bible School, Stewarton, Scotland;http://www.rmplc.co.uk/eduweb/sites/ sbs777/vital/evolution.html 
60.Alexander I. Oparin, Origin of Life, New York: Dover Publications, 1936, (reprint 1953), p. 196. 
61.Robert Shapiro, Origins: A Skeptic's Guide to the Creation of Life on Earth, New York: Summit Books, 1986, p. 99. 
62.Klaus Dose, "The Origin of Life: More Questions Than Answers," Interdisciplinary Science Reviews, Vol. 13, no. 4, 1988, p. 348. 
63. Mere Creation, Edited By William A. Dembski, Illinois: Intervarsity Press, 1998, pp. 116-119. 
64.Stephen C. Meyer, "The Origin of Life and the Death of Materialism," reprinted from the Intercollegiate Review 31, No. 2, (Spring 1996). 
65."New Evidence on Evolution of Early Atmosphere & Life," Bulletin of the American Meteorological Society, Vol. 63, November 1982, pp. 1328-1330. 
66.Richard B. Bliss, Gary E. Parker, Duane T. Gish, Origin of Life (3rd edition), C.L.P. Publications, 1990, pp. 14-15. 
67."Life's Crucible," Earth, February 1998, p. 34 (emphasis added). 68. "The Rise of Life on Earth," National Geographic, March 1998, p. 
69.Wendell R. Bird, The Origin of Species Revisited, Nashville: Thomas Nelson Co., , 1991, p. 325. 
70.The chemist Richard E. Dickinson explains the reason for this: "If protein and nucleic acid polymers are to consist of primary molecules, then one water molecule has to be released when each monomer binds to the polymer chain. It is hard to see how polymerization can proceed when the presence of water causes polymers to break down rather than form." 
71.Richard B. Bliss, Gary E. Parker, Origin of Life, 1979, p. 25. 
73.P. W. Fox, K. Harada, G. Kramptiz, G. Mueller, "Chemical Origin of Cells," Chemical Engineering News, 22 June, 1970, p. 80. 