In order to survive, plants need to carry out photosynthesis, and for that they need the water and minerals they take from the soil. To meet these needs, they require the roots which drill under the ground. The job of the roots is to spread rapidly underground like a net and draw up water and minerals. As well as this, plant roots, despite their delicate structure, enable plants which can weigh up to tons to hold on to and fix themselves in the soil. The soil-gripping nature of roots is most important, because it prevents landslides and the fertile upper layers of soil being washed away by the rain, and other unwanted occurrences that can adversely affect human life.
 |
Roots need no equipment for all this. They have no engine to provide the power to start the process of water-drawing. Neither is there any equipment to pump the water and minerals to the stem, metres away. But roots can spread over a wide area and draw water. So, how do they do it?
A typical red maple tree growing in a humid climate may lose as much as 200 liters of water per day. This represents a serious loss for the tree. This water needs to be replaced immediately if the plant is to survive. Thanks to the flawless root system plants have, every drop of water which evaporates is replaced.31
The roots, which spread down into the depths of the earth, send the water and minerals which the plant needs right up to the leaves, through the stem and branches. The roots' drawing of water from under the ground closely resembles a drilling technique. The ends of the roots keep looking for water in the depths of the soil until they find it. Water enters the root through an external membrane and capillary cells. It then passes through the cells to the stem tissue. From there it is transported to every part of the plant.
This process which the plant carries out so perfectly is, in fact, a very complicated one. So much so that the secret of the system is still not completely known, even in these days of space-age technology. The existence of this sort of "pressure tank" system was discovered in trees some 200 years ago. Yet no law has yet been discovered to definitively explain how this movement of water, against gravity, actually comes about. All that scientists have been able to do on this subject is put forward a number of theories about certain mechanisms. Those which have been demonstrated in experiments are thought of as valid to some extent. The outcome of all these scientists' efforts is the recognition of the perfection of the pressure tank system. Such a technology, packed into a tiny space, is just one of the proofs of the incomparable intelligence of the designer of the system. The water transport system in trees, and everything else in the universe, were created by God.
The Water Transport System | ||
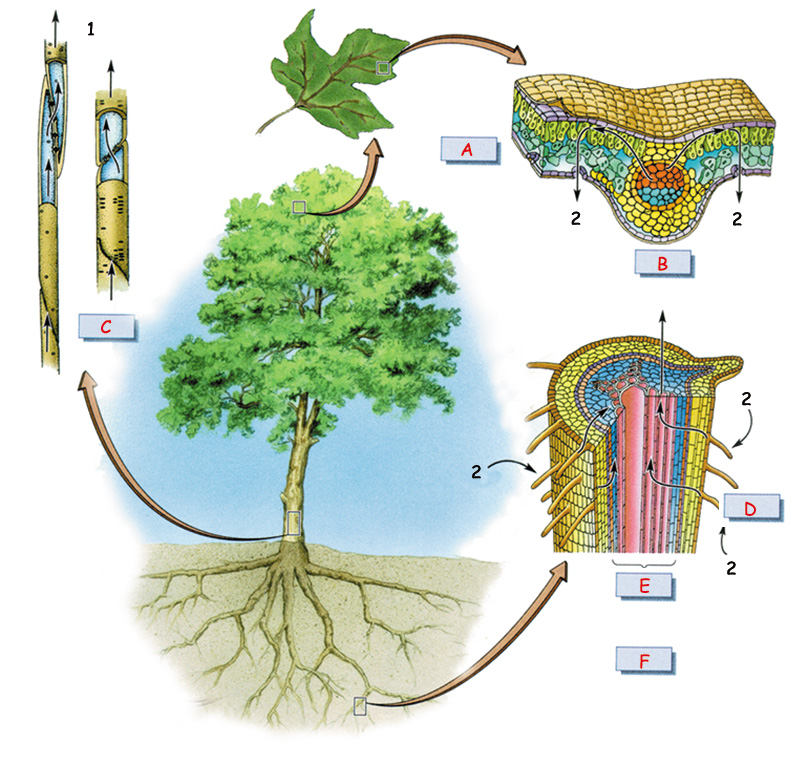 | ||
| A. Leaf | D. Root hairs | 1. Transport pipes |
When the internal pressure in root cells is lower than the outside pressure, plants take in water from outside. Another way of putting it is that they take water from outside only when they need it. The most important factor establishing this is the amount of pressure produced by the water in the roots. This pressure has to be balanced with that outside. For this to happen, the plant needs to take in water from the outside when the amount of internal pressure falls. When the opposite happens, when the inside pressure is higher than the outside, the plant gives off water from inside itself by means of its leaves to re-establish the balance.
If the level of the water in the soil were slightly higher than normal, the plant would continually take in water, because the external pressure was higher, and this would eventually damage it. If it were a little lower, on the other hand, the plant cell could never take in water from the outside because the external pressure would be low. It would even have to give off water to maintain the pressure balance. In either case the plant would dry up and die.
This shows to us that plant roots contain a balance-control mechanism to enable them to regulate the level of pressure needed at a precise moment, neither more nor less.
The General Structure of the Root End | |
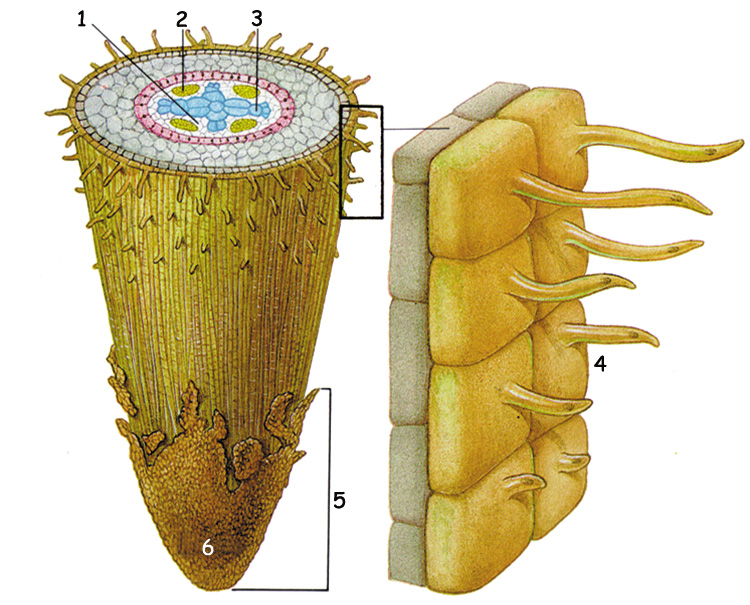 | 1. Cambium 4. Root hairs On the left page can be seen a detailed plan of all the elements in a plant's transport system. The roots carry the water they absorb from the soil to the steele, where it enters the vascular system in the stem. Through the vascular system, water and nutrients make a trip upward for metres in the stem, tirelessly, right up to the farthest leaves. The system, which starts at the roots and goes as far as the leaves is unarguably the product of a most superior planning. This planning belongs without doubt to God, the Creator of everything. The picture to the side shows the general structure of a growing root tip and a close up of the root hairs which lie just behind the tip. |
The cells in the roots of a plant select particular ions from the soil to use in cell reactions. Plant cells can easily take these ions inside themselves, despite the internal concentration of some ions in the plant being a thousand times greater than that in the soil solution. So, this is a most important process.32
Under normal conditions, a transfer of materials will occur from an area with a higher concentration to one with a lower concentration. But as we have seen, just the opposite takes place in the roots' absorbing ions from the soil. For this reason the process requires quite substantial amounts of energy.
Two factors influence the passage of the ions through the cell membrane: the membrane's permeability and the concentration of the ions on either side of the membrane.
Let us examine these two factors by asking some questions about them. What does a plant's choosing the required elements from those in the soil actually mean? Let us first take the concept of "requirements." A root cell has to know all the elements in the plant, one by one, to meet its requirements. It has to establish which of all the elements it knows are lacking in all parts of the plant and identify them as needs. Let us ask another question. How is an element known? If the soil is not in a pure state, in other words if there are other elements mixed up in it, what has to be done to distinguish one element from all the rest?
Will it be possible for someone to tell which is which if elements such as iron, calcium, magnesium, and phosphorus are put in front of him all mixed up? How can he tell them apart? If he has received training in the subject, he may be able to identify some of them. It will be impossible for him to identify the rest, however. So how do plants make the distinction? Or rather, how is it possible for a plant to know elements by itself, and to find those ones most useful for it? Is it possible that such a process should have been carried out in the right way every time for millions of years by chance? In order to think about all of these questions-to which the answer is "Impossible!"-in a more detailed and deeper way, let us examine what kind of selective property roots possess and what happens at the time of selection.
 | Let us imagine that the minerals in the picture were put in front of us and we were asked to choose which of them were necessary for our bodies. It is impossible for anybody who has not had special training to do this. Whereas plants have been selecting and using only those elements they need from all those in the soil for millions of years. Of course it is God, their Creator, who makes it possible for plants to carry out this process, which for human beings is impossible. |
Let us review our chemical knowledge regarding the elements and minerals which appear in many forms in nature. Where are they found? Which substances go into which groups? What differences are there between them? What experiments or observations are required to understand what each one is? Can the fastest results be arrived at by chemical or physical methods in these experiments? If we just look at things from the physics point of view can we make a proper classification of these substances if they are put on a table in front of us? Can we distinguish minerals by their colour or form?
We could go on. And the answer to all of the above questions is more or less the same. Unless someone is an expert in the field, partial or inadequate knowledge left over from school or university will not lead a person to an accurate solution. In order to classify our knowledge of minerals, let us this time take examples from the human body.
There is a total of three kilograms of minerals in our bodies. Parts of them are essential for our health, and they are all present in the necessary quantities. For example, if we had no calcium in our bodies, our teeth and bones would lose their hardness. If there were no iron, oxygen could not reach our tissues, because we would have no haemoglobin. If we had no potassium and sodium, our cells would lose their electrical charges and we would rapidly age.
Minerals are present in the soil in the same way as in the human body. Their quantities, functions, and the forms in which they are found in the soil are all different, and many living things make use of these minerals. In plants, for instance, systems have been set up so that they can easily take the elements they need from the soil. There being different fields of use for them in their structures, all the elements have to go to different parts of the plant after they are absorbed. They all have different tasks.
Elements Required by Plants | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This table shows the elements plants need, where plants take these elements from, and how they are used. Plants only take and use the 16 elements they need from among all those present in the soil. These processes, which even people who study them find hard to understand, are carried out by plants, thanks to the inspiration of God. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In order to live healthily, a plant needs such basic elements as nitrogen, phosphorus, potassium, calcium, magnesium and sulphur. While plants can take most of these substances directly from the soil, the situation is different with nitrogen. Nitrogen makes up almost 80% of the atmosphere by volume, however, it cannot be obtained or "fixed" directly from the atmosphere by green plants. The plants meet their nitrogen need by absorbing from the soil the nitrates processed by the soil bacteria.
Other elements, too, are necessary for healthy development. But these are needed in quite small quantities. This group includes iron, chlorine, copper, manganese, zinc, molybdenum, and boron.
In addition to these 13 minerals, plants also need the three basic building blocks of oxygen, hydrogen, and carbon, and get them from the carbon-dioxide, oxygen, and water in the atmosphere. All plants need this total of 16 elements.
If these elements are taken in in too great or too small quantities, various deficiencies arise in the plant.
Carbon and Nitrogen Cycle | |
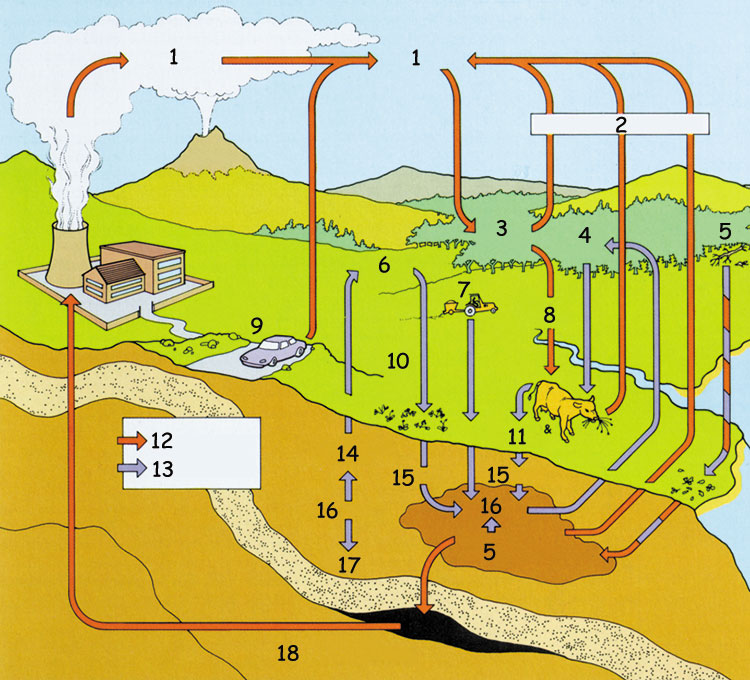 | |
| 1- Carbon dioxide | 10- Nitrogenfixing bacteria |
| The most important factor contributing to the carbon and nitrogen cycle in the environment, as outlined in the above picture, is without doubt plant life. The nitrogen in the air cannot be taken in directly by people and animals. When the nitrogen is passed to the soil, the ammonia released is then oxidized by soil bacteria to nitrates, and in this form it can be reabsorbed by plant roots. People and animals then meet their nitrogen needs by eating the plants. | |
For example, too much nitrogen from the soil leads to brittle growth especially under high temperatures and succulent growth, while too little can lead to yellowing, red and purple patches, reduced lateral bud, and older growth. Phosphorus deficiency causes reduced growth, browning or purpling in foliage in some plants, thin stems, reduced lateral bud breaks, loss of lower leaves and reduced flowering. Phosphorus is a very important element for the growth of young plants and seed production. In short, the existence of these ions and their being taken in from the soil in the required quantities are essential for healthy plant growth.33
What would happen if plants did not possess this ion-selection mechanism? What would happen if plants took in all kinds of minerals, not just the ones they need, or took in too many or too few minerals? There is no doubt that in that event there would be serious disruptions to the perfect balance in the world.
Do they not look at the sky above them?- How We have made it and adorned it, and there are no flaws in it? And the earth- We have spread it out, and set thereon mountains standing firm, and produced therein every kind of beautiful growth (in pairs)- To be observed and commemorated by every devotee turning (to Allah). (Surah Qaf, 6-8)
31. Milani, Bradshaw, Biological Science, A molecular Approach, D.C.Heath and Company, Toronto, p.430
32. Malcolm Wilkins, Plantwatching, New York, Facts on File Publications, 1988, p.119
33. http://ag.arizona.edu/pubs/garden/mg/botany/macronutrient.html