Some of the vital components of the blood are proteins. Through the circulatory network reaching all points in the body, proteins in the blood have the means to reach every cell where they are needed. For example, the protein hemoglobin carries oxygen to the tissues, and the protein called transpherine in the blood carries iron. Immunoglobins are proteins that protect the body against bacteria and viruses. Fibrinogen and thrombin cause the blood to clot. Insulin is a variety of protein that regulates sugar metabolism in the body. All of these essential proteins reach the tissues by means of the bloodstream.
 |
| The elegance of the way the hemoglobin system functions is simply astounding, and a source of wonder to everyone who is familiar with its intricate ingenuity. —Michael Denton |
 |
| Blood cells perform analyses far more expertly than any chemist or biologist, and carefully transmit the substances needed by the cells to every point in the body. Unconscious blood cells cannot take such vitally important decisions themselves. That decision is inspired in them by our Lord. |
Albumin, one of the transport proteins in the blood, attaches to fats such as cholesterol, hormones, poisonous bile and drugs such as penicillin. Later, the blood moves it through the body to the liver, where it deposits the toxins it collects to be neutralized, and carries nutrients and hormones to where they are needed. How can a molecule like albumin, composed of atoms with no knowledge or consciousness, distinguish between fats, toxins, drugs and nutrients?
Moreover, how is it able to deposit the substances it carries in the liver, gall bladder and stomach, without ever making a mistake, and in the needed quantities? If you examine the toxins, drugs and nutrients carried in the blood under a microscope, you could never tell one from another without studying clinical biochemistry. You could never tell how much of each needs to be deposited at which organ.
Molecules know this information, which most people without special training do not possess, and they have been performing their function in the body flawlessly ever since the first human being appeared. No doubt the display of consciousness by a collection of atoms is possible only through Allah's infinite might and knowledge.
 |
| The hemoglobin molecule shown has a very special structure created to carry oxygen in the blood, and one that scientists speak of with amazement: "It would seem that in designing an oxygen-transporting molecule from first principles we are led inevitably to a molecule very like hemoglobin. The evidence is consistent with the possibility that hemoglobin is the ideal and unique respiratory pigment for metabolically active air-breathing organisms ... The elegance of the way the hemoglobin system functions is simply astounding, and a source of wonder to everyone who is familiar with its intricate ingenuity." (Michael J. Denton, Nature's Destiny, The Free Press, 1998, p. 202.) |
The most important property of the protein hemoglobin in the blood's erythrocyte cells is its ability to trap oxygen atoms. Hemoglobin carefully selects oxygen molecules from among the millions of molecules in the blood. However, a hemoglobin molecule attaching to an oxygen molecule would be oxidized and lose its function. For that reason, hemoglobin traps the oxygen molecule with a special technique, the result of a special Creation—not touching it at all, as if it were using tongs.
Hemoglobin consists of the assembly of four different proteins, in which there are special iron atom-bearing sections. These regions that carry iron atoms are known as heem (or haem) groups. The iron atoms in these heem groups are the special tongs by which oxygen is held. Each heem group can hold one oxygen molecule.64 Special folds and angles within the molecule let the heem groups trap oxygen without touching it and carry it to the tissues. These angles change in specific proportions during the binding process. 65
After the first heem group has trapped oxygen, the hemoglobin structure changes, and greatly facilitates the trapping of oxygen by the other heem groups.66 If the hemoglobin combines directly with the oxygen during the trapping process—if oxidized, in other words—then a disorder known as methemoglobinemia results,67 causing the skin to lose its color and turn blue, accompanied by shortness of breath and weakening of the mucous membranes. 68
As a result of the special Creation of the hemoglobin molecule, however, these molecules regularly carry 600 liters of oxygen every day to the 100 trillion or so cells in your body. It is impossible for hemoglobin to know that oxygen will damage it, to take the appropriate precautions by making a special arrangement in its own structure and to know that it must carry oxygen to every cell in the body. The molecule in question is nothing more than a collection of unconscious atoms. However, our Omniscient Lord, Creator of all, has created the hemoglobin molecule to protect it from oxygen's harmful effects, and reveals to us the fine detail of His Creation. In his book Nature's Destiny, the famous microbiologist Michael Denton describes the flawless Creation in hemoglobin:
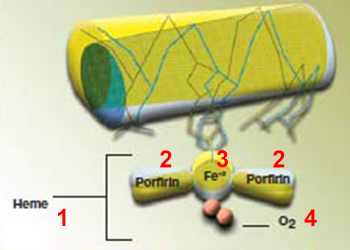 | |
| 1. Heme | 3. Fe+2 |
| The way that hemoglobin knows it will be damaged if it binds to oxygen and takes the requisite precautions is an indication that Allah enfolds all places with His knowledge. In carrying an oxygen molecule to the cells, the hemoglobin molecule does not bind to it fully, but grasps it from one end, as if it were using tongs. Oxygen poisons the molecules to which it attaches and leads them to lose their function. To eliminate that danger, hemoglobin employs a very special method. Yet a molecule lacks the consciousness to recognize danger, and the reason with which to take precautions. All these details display our Lord's superior knowledge. | |
As the efficient transport of oxygen is essential to the viability of any large active organism with a high metabolic rate, a molecule with properties of hemoglobin would seem to be essential. Might there be any alternatives to hemoglobin? None of the many other oxygen-carving molecules which occur in the blood of invertebrates, such as the copper-containing proteins of the molluscs, come close to the efficiency of hemoglobin in transporting oxygen in blood. As Ernest Baldwin commented, "Mammalian haemoglobin is far and away the most successful of the respiratory pigments from this point of view" ... The evidence is consistent with the possibility that hemoglobin is the ideal and unique respiratory pigment for metabolically active air-breathing organisms ... 69
As Michael Denton states here, hemoglobin is the most ideal form of transport. The way that a mass of molecules can distinguish one molecule from another in the darkness of the body, in an area very large compared with its own size, and attach itself to that molecule in the most appropriate way reveals one of the proofs of Almighty Allah's infinite knowledge.
The way that cells bind to one another selectively is another of their most important features. According to the biologist John P. Trinkhaus:
The adhesions that cells make with one another lie at the very basis of multicellularity. The form and functioning of all creatures that consist of more than one cell depend on their cells adhering firmly to one another and to the extracellular materials that intervene. 70
The way cells selectively attach to other cells around them also depends on the properties of the cell membrane. With such features as viscosity, density and electrical activity, the double-layered phospholipids cell membrane is the most suitable structure for making life possible. The phenomena taking place in the cell membrane should require consciousness and intellect. How does a cell membrane, a combination of unconscious molecules, recognize another cell? How does it know that it must attach to other cells to form an organ, and how it can do so? This property of the cell is one of the examples of our Lord's dominion over living things.
64. Helena Curtis, N. Sue Barnes, Biology, New York: Worth Publishers, Inc, , 1989, p. 51.
65. Prof. Dr. Engin Gozukara, Biochemistry, Nobel Medical Houses, 1997, 3rd printing, Vol 1, p. 176.
66. Albert L. Lehninger, David L. Nelson, Michael M. Cox, Principles of Biochemistry, 2nd printing, New York: Worth Publishers, , p. 189.
67. http://www.britannica.com/bcom/eb/article/7/0,5716,53637+1+52330,00.html?query=methemoglobinemia
68. Reto Kohler, "Chuck it out," New Scientist, Vol. 166, No. 2242, 10 July 2000, p. 28.
69. Michael Denton, Nature's Destiny, New York: Free Press, , pp. 201-202.
70. J. P. Trinkaus, Cells into Organs, , Englewood Cliffs, NJ: Prentice-Hall, 1984, p. 69.