Chapter 3: The Second Step on the Path to Matter: Molecules
What is it that makes the objects you see in your surroundings different from each other? What is it that discriminates their colours, shapes, smells, and tastes? Why is one substance soft, another hard, and yet another fluid? From what you have read so far, you may answer these questions saying, "The differences between their atoms do this". Yet, this answer is not sufficient, because if the atoms were the cause for these differences, then there would have to be billions of atoms bearing different properties from each other. In practice, this is not so. Many materials look different and bear different properties although they contain the same atoms. The reason for this is the different chemical bonds the atoms form among them to become molecules.
On the way to matter, molecules are the second step after atoms. Molecules are the smallest units determining the chemical properties of matter. These small bodies are made up of two or more atoms and some, of thousands of groups of atoms. Atoms are held together inside molecules by chemical bonds determined by the electromagnetic force of attraction, which means that these bonds are formed on the basis of the electrical charges of the atoms. The electrical charges of atoms, in turn, are determined by the electrons on their outermost shell. The arrangement of molecules in different combinations give rise to the diversity of matter we see around us. The importance of the chemical bonds that lie at the heart of the diversity of matter come forward at this very point.
Chemical Bonds
As explained above, chemical bonds are formed through the motion of electrons in the outermost electron shells of the atoms. Each atom has a tendency to fill up its outermost shell with the maximum number of electrons it may shelter. The maximum number of electrons the atoms can hold in their outermost shells is 8. To do this, atoms either receive electrons from other atoms to complete the electrons in their outermost shells to eight, or if they have lesser electrons in their outermost shells, then they give these to another atom, making a sub-shell that had previously been completed in their outermost orbits. The tendency of the atoms to exchange electrons constitutes the basic inciting force of the chemical bonds they form between each other.
This driving force, that is, the objective of the atoms to raise the number of electrons in their outermost shells to maximum, causes an atom to form three types of bonds with other atoms. These are the ionic bond, covalent bond and metallic bond.
Commonly, special bonds categorised under the general title of "weak bonds" act between molecules. These bonds are weaker than the bonds formed by atoms to constitute molecules because molecules need more flexible structures to form matter.
Let us now, in brief, see the properties of these bonds and how they are formed.
Ionic Bonds
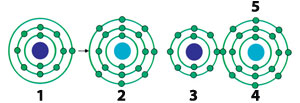 | |
| 1) Sodium atom | 4) Chlorine ion |
| The sodium atom gives its outermost electron to a chlorine atom and becomes positively charged. Receiving the electron, the chlorine atom becomes negatively charged. The two form an ionic bond through these two opposite charges attracting each other.24 | |
Atoms combined by this bond swap electrons to complete the number of electrons in their outermost shells to eight. Atoms having up to four electrons in their outermost shells give these electrons to the atom with which they are going to combine, that is, with which they will bond. Atoms having more than four electrons in their outermost shells receive electrons from the atoms with which they will bond. Molecules formed by this type of bond have crystal (cubic) structures. Familiar table salt (NaCl) molecules are among substances formed by this bond. Why do atoms have such a tendency? What would happen if they did not have it?
Until today, the bonds formed by atoms could be defined only in very general terms. It has not yet been understood why atoms adhere to this principle. Could it be that atoms decide by themselves that the number of electrons in their outermost shells should be eight? Definitely not. This is such decisive behaviour that it goes beyond the atom, because it has no intellect, will, or consciousness. This number is the key in the combination of atoms as molecules that constitute the first step in the creation of the matter, and eventually, the universe. If atoms did not have such a tendency based on this principle, some vital molecules would not exist. Yet, from the first moment they were created, atoms have been serving in the formation of molecules and matter in a perfect manner thanks to this tendency.
Covalent Bonds
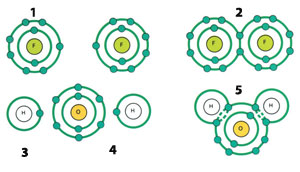 | |
| 1) Fluorine atom | 3) Hydrogen atom |
| Some atoms form new molecules by covalent bonding, sharing the electrons in their outer orbits.25 | |
Scientists who studied the bonds between atoms faced an interesting situation. While some atoms swap electrons for bonding, some of them share the electrons in their outermost shells. Further research revealed that many molecules that are of critical importance for life owe their existence to these 'covalent' bonds.
Let us give a simple example to explain covalent bonds better. As we mentioned previously on the subject of electron shells, atoms can carry a maximum of two electrons in their innermost electron shells. The hydrogen atom has a single electron and it has the tendency to increase the number of its electrons to two to become a stable atom. Therefore, the hydrogen atom forms a covalent bond with a second hydrogen atom. That is, the two hydrogen atoms share each other's single electron as a second electron. Thus, the H2 molecule is formed.
Metallic Bonds
If a large number of atoms come together by sharing each others' electrons, this is called a "metallic bond". Metals like iron, copper, zinc, aluminium, etc., that form the raw material of many tools and instruments we see around us or use in daily life, have acquired a substantial and tangible body as a result of the metallic bonds formed by the atoms constituting them.
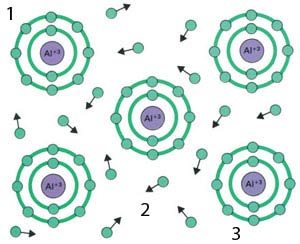 | 1) Metallic Bonding |
| The bonds between metal atoms are very different from other forms of chemical bonding – each metal atom contributes its outer electrons to a common pool. This "sea of electrons" explains a key property of metals – their ability to conduct electricity.26 |
Scientists are not able to answer the question as to why electrons in the electron shells of the atoms have such a tendency. Living organisms, most interestingly, owe their existence to this tendency.
The Next Step: Compounds
Do you wonder how many different compounds these bonds can form?
In laboratories, new compounds are produced everyday. Currently, it is possible to talk about almost two million compounds. The simplest chemical compound can be as small as the hydrogen molecule, while there are also compounds made up of millions of atoms.27
How many different compounds can an element form at most? The answer to this question is quite interesting because, on the one hand, there are certain elements that do not interact with any others (inert gases), while, on the other hand, there is the carbon atom that is able to form 1,700,000 compounds. As stated above, the total number of compounds is about two million. 108 elements out of the total of 109 form 300,000 compounds. Carbon, however, forms 1,700,000 compounds all by itself in a most amazing fashion.
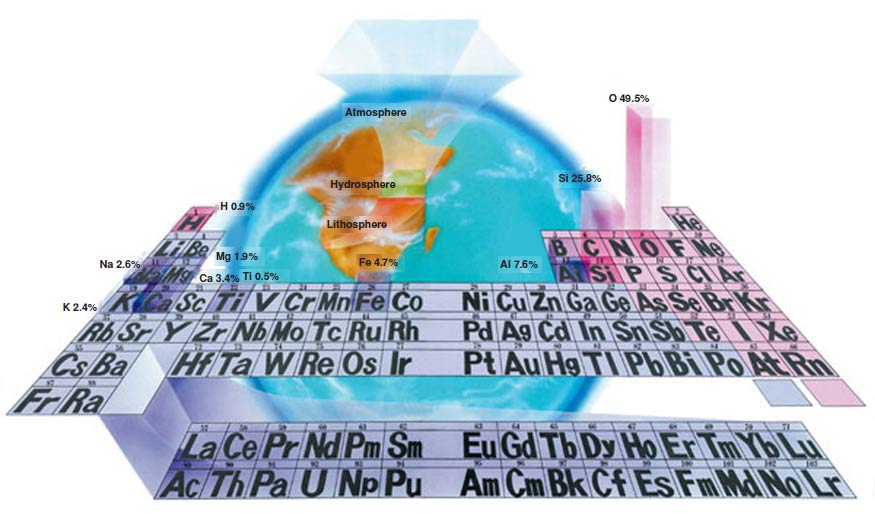 |
| The Raw Materials of the Universe and the Periodic Table: 92 elements found freely in nature and 17 elements formed artificially in laboratories or in nuclear reactions are arranged in a table called the "Periodic Table" according to the number of their protons. At first look, the Periodic Table may appear to be a bunch of boxes containing one or two letters with numbers at the top and bottom corners. Most interestingly, however, this table accommodates the elements of the entire universe including the air we breathe, as well as of our bodies. |
The Building Block of Life: the "Carbon" Atom
 |
| Carbon atom |
Carbon is the most vital element for living beings, because all living organisms are constructed from compounds of carbon. Numerous pages would not be enough to describe the properties of the carbon atom, which is extremely important for our existence. Nor has the science of chemistry yet been able to discover all of its properties. Here we will mention only a few of the very important properties of carbon.
Structures as diverse as the cell membrane, the horns of an elk, the trunk of a redwood, the lens of the eye, and the venom of a spider are composed of carbon compounds. Carbon, combined with hydrogen, oxygen, and nitrogen in many different quantities and geometric arrangements, results in a vast assortment of materials with vastly different properties. So, what is the reason for carbon's ability to form approximately 1.7 million compounds?
One of the most significant properties of carbon is its ability to form chains very easily by lining carbon atoms up one after another. The shortest carbon chain is made up of two carbon atoms. Despite the unavailability of an exact figure on the number of carbons that make up the longest carbon chain, we can talk about a chain with seventy links. If we consider that the atom that can form the longest chain after the carbon atom is the silicon atom forming six links, the exceptional position of the carbon atom will be better understood.28
The reason for carbon's ability to form chains with so many links is because its chains are not exclusively linear. Chains may be branched, as they may also form polygons.
At this point, the form of the chain plays a very important role. In two carbon compounds, for example, if the carbon atoms are the same in number yet combined in different forms of chains, two different substances are formed. The abovementioned characteristics of the carbon atom produce molecules that are critical for life.
Some carbon compounds' molecules consist of just a few atoms; others contain thousands or even millions. Also, no other element is as versatile as carbon in forming molecules with such durability and stability. To quote David Burnie in his book Life:
Carbon is a very unusual element. Without the presence of carbon and its unusual properties, it is unlikely that there would be life on Earth.29
Three Similar Molecules Result: Three Very Different Substances |
| Even a difference in a few atoms between molecules leads to very different results. For instance, look carefully at the two molecules written below. They both seem very similar except for very small differences in their carbon and hydrogen components. The result is two totally opposite substances: C18H24O2 and C19H28O2Can you guess what these molecules are? Let us tell you immediately: the first is oestrogen, the other is testosterone. That is, the former is the hormone responsible for female characteristics and the latter is the hormone responsible for male characteristics. Most interestingly, even a difference of a few atoms can cause sexual differences. C6H12O2Doesn't this molecule look very much alike the oestrogen and testosterone hormone molecules? So, what is this molecule, is it another hormone? Let us answer right away: this is the sugar molecule. From the examples of these three molecules made up of elements of the same type, it is very clear how diverse the substances are that the difference in the number of atoms may produce. On the one hand, there are the hormones responsible for sexual characteristics, while on the other hand, there is sugar, a basic food. |
Concerning the importance of carbon for living beings, the British chemist Nevil Sidgwick writes in Chemical Elements and Their Compounds:
Carbon is unique among the elements in the number and variety of the compounds which it can form. Over a quarter of a million have already been isolated and described, but this gives a very imperfect idea of its powers, since it is the basis of all forms of living matter. 30
 |
| Diamond, which is a very valuable stone, is a derivative of carbon, which is otherwise commonly found in nature as graphite. |
The class of compounds formed exclusively from carbon and hydrogen are called "hydrocarbons". This is a huge family of compounds that include natural gas, liquid petroleum, kerosene, and lubricating oils. The hydrocarbons ethylene and propylene form the basis of the petrochemical industry. Hydrocarbons like benzene, toluene, and turpentine are familiar to anyone who's worked with paints. The naphthalene that protects our clothes from moths is another hydrocarbon. Hydrocarbons combined with chlorine or fluorine form anaesthetics, the chemicals used in fire extinguishers and the Freons used in refrigeration.
As the chemist Sidgwick stated above, the human mind is insufficient to fully understand the potential of this atom that has only six protons, six neutrons and six electrons. It is impossible for even a single property of this atom, which is vital for life, to form by chance. The carbon atom, like everything else, has been created by Allah perfectly adapted for the bodies of living beings, which Allah encompasses down to their very atoms.
What is in the heavens and in the earth belongs to Allah. Allah encompasses all things. (Surat an-Nisa': 126)
What Would Happen If Every Atom That Stood Close Together Immediately Reacted? | |
| We just said that the whole universe is formed by the interaction of the atoms of 109 different elements. Here, there is a point that needs to be mentioned, which is that a very important condition must be fulfilled for the reaction to start.
For instance, water does not form whenever oxygen and hydrogen come together and iron does not rust away as soon as it comes in contact with air. If it did so, iron, which is a hard and shiny metal, would be transformed into ferrous oxide, which is a soft powder, in a few minutes. No such thing as a metal would be left on earth and the order of the world would be greatly disturbed. If atoms that happened to be placed close to each other at a certain distance had united immediately without the fulfilment of certain conditions, atoms of two different substances would have interacted right away. In that case, it would be impossible even for you to sit on a chair, because the atoms forming the chair would immediate react with the atoms forming your body and you would become a being between chair and human (!). Of course, in such a world, life would be out of the question. How is such an end avoided? To give an example, hydrogen and oxygen molecules react very slowly at room temperature. That means that water forms very slowly at room temperature. Yet, as the temperature of the environment rises, the energies of molecules also increase and reaction is accelerated, and thus water is formed more rapidly. The minimum amount of energy required for molecules to react with each other is called the "activation energy". For instance, in order for hydrogen and oxygen molecules to react with each other to form water, their energy has to be higher than the activation energy. Just consider. If the temperature on earth were a little higher, the atoms would react too rapidly, which would destroy the equilibrium in nature. If the opposite were true, that is the temperature on earth were lower, then atoms would react too slowly, which would again disturb the equilibrium in nature. As this clarifies, the distance of the earth from the sun is just appropriate to support life on earth. Certainly, the delicate balances required for life do not end there. The inclination in the axis of the earth, its mass, surface area, the proportion of the gases in its atmosphere, the distance between the earth and its satellite, the moon, and many other factors have to be precisely at their present values so that living beings can survive. This points to the fact that all these factors could not have formed progressively by chance and that they were all created by Allah, the Owner of Supreme power, Who knows all the properties of living beings. Typically, the role of science during these processes is just to name the laws of physics that it observes. As we explained in the beginning, in the case of such phenomena, questions like "what?", "how?", and "in what way?" fade into insignificance. What we can reach by these questions are only the details of an already existing law. The main questions that should be asked are "why?" and "by whom was this law created"? The answer to these questions remains an enigma for scientists who blindly adhere to their materialist dogmas. At this point, where materialists reach a deadlock, the picture is very clear for a person who looks at events by using his mind and conscience. The flawless balances in the universe, which cannot be explained as coincidences, have been brought about at the bidding of a supreme mind and will, as stated in the verse, "Allah takes account of everything." (Surat an-Nisa: 86), and He created everything according to a very precise calculation, order and equilibrium. |
Intermolecular Bonds: Weak Bonds
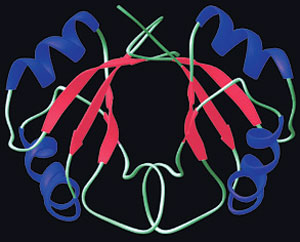 |
| The sequence of the amino acids and the three-dimensional shape determine the function of the protein in the body. Weak bonds between molecules form these structures. |
The bonds combining the atoms in molecules are much stronger than these weak intermolecular bonds. These bonds can help the formation of millions, and even billions of kinds of molecules.
Well, how do molecules combine to form matter?
Since molecules become stable after their formation, they no longer swap atoms.
So, what holds them together?
In an effort to answer this question, chemists produced different theories. Research showed that molecules are able to combine in different ways depending on the properties of the atoms in their composition.
These bonds are very important for organic chemistry, which is the chemistry of living beings, because the most important molecules constituting life are formed due to their ability to form these bonds. Let us take the example of proteins. The complex three-dimensional shapes of proteins, which are the building blocks of living things, are formed thanks to these bonds. This means that the weak chemical bond between molecules is at least as necessary as the strong chemical bond between atoms for the formation of life. Certainly, the strength of these bonds must be of a certain measure.
We can continue with the protein example. Molecules called amino acids combine to form proteins, which are much larger molecules. The atoms forming amino acids are combined by covalent bonds. Weak bonds combine these amino acids in such a way as to produce three-dimensional patterns. Proteins can function in living organisms only if they have these three dimensional patterns. Therefore, if these bonds did not exist, neither would the proteins, or, therefore, life exist.
The "hydrogen" bond, a type of weak bond, plays a major role in the formation of materials that bear great importance in our lives. For instance, the molecules forming water, which is the basis of life, are combined by hydrogen bonds.
 |
| Do you not see that Allah sends down water from the sky and forthwith the earth is covered in green? Allah is All-Subtle, All-Aware. (Surat al-Hajj: 63) |
A Miracle Molecule: Water
A liquid specifically chosen for life – "water" – covers two-thirds of our earth. The bodies of all living beings on the earth are formed of this very special liquid at a ratio ranging between 50%-95%. From bacteria living in springs with temperatures close to the boiling point of water, to some special mosses on melting glaciers, life is present everywhere where there is water, no matter at what temperature. Even in a single droplet hung on a leaf after rain, thousands of microscopic living organisms emerge, reproduce, and die.
How would the earth look if there were no water? Certainly, everywhere there would be desert. There would be abysses and horrific pits, in place of seas. The sky would seem cloudless and have a strange colour.
In fact, it is extremely difficult for water, the basis of life on earth, to form. First, let us imagine that hydrogen and oxygen molecules, which are the components of water, are put in a glass bowl. Let us leave them in the bowl for a very long time. These gases may still not form water even if they remain in the bowl for hundreds of years. Even if they do, it would not be more than a very small amount at the very bottom of the bowl and that would happen in a very slow fashion, maybe over thousands of years.
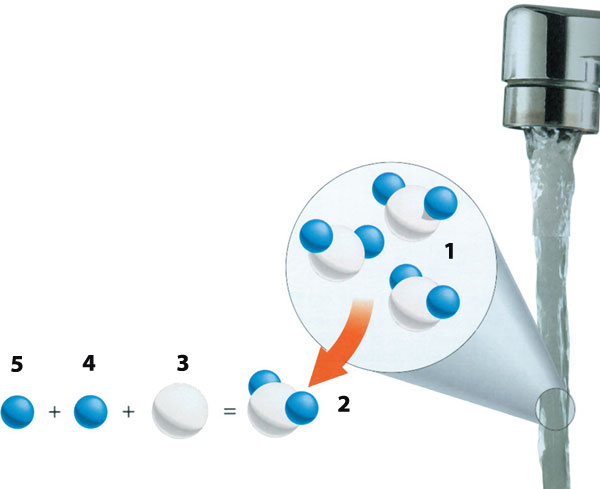 | ||
| 1) Water Molecule | 3) Oxygen | 5) Hydrogen |
The reason why water forms so slowly under these circumstances is temperature. At room temperature, oxygen and hydrogen react very slowly.
Oxygen and hydrogen, when free, are found as H2 and O2 molecules. To combine to form the water molecule, they must collide. As a result of this collision, the bonds forming the hydrogen and oxygen molecules weaken, leaving no hindrance for the combination of oxygen and hydrogen atoms. Temperature raises the energy and therefore, the speed of these molecules, resulting in an increase in the number of collisions. Thus, it accelerates the course of the reaction. However, currently, no temperature high enough to form water exists on earth. The heat required for the formation of water was supplied during the formation of the earth, which resulted in the emergence of so much water as to cover three quarters of the earth's surface. At present, water evaporates and rises to the atmosphere where it cools and returns to the earth in the form of rain. That is, there is no increase in the quantity; only a perpetual cycle.
The Miraculous Properties of Water
 |
| If water did not have the property of freezing from the surface downwards, a major portion of the seas would be frozen within a year and life in the sea would be endangered. |
Water has many exceptional chemical properties. Every water molecule forms by the combination of hydrogen and oxygen atoms. It is quite interesting that these two gases, one combustive and the other combustible, combine to form a liquid, and most interestingly, water.
Now, let us briefly see how water is formed chemically. The electrical charge of water is zero, that is, it is neutral. Yet, due to the sizes of the oxygen and hydrogen atoms, the oxygen component of the water molecule has a slightly negative charge and its hydrogen component has a slightly positive charge. When more than one water molecule come together, positive and negative charges attract each other to form a very special bond called "the hydrogen bond". The hydrogen bond is a very weak bond and it is incomprehensibly short-lived. The duration of a hydrogen bond is approximately one hundred billionth of a second. But as soon as a bond breaks, another one forms. Thus, water molecules adhere tightly to each other while also retaining their liquid form because they are combined with a weak bond.
Hydrogen bonds also enable water to resist temperature changes. Even if air temperature increases suddenly, water temperature increases slowly and, similarly, if air temperature falls suddenly, water temperature drops slowly. Large temperature changes are needed to cause considerable changes in water temperature. The significantly high thermal energy of water has major benefits for life. To give a simple example, there is a great amount of water in our bodies. If water adapted to the sudden vicissitudes of temperature in the air at the same rate, we would suddenly develop fevers or freeze.
 |
| Because the density of frozen water is less than water in liquid form, ice floats on water. |
By the same token, water needs a huge thermal energy to evaporate. Since water uses up a great deal of thermal energy while evaporating, its temperature drops. To give an example, again from the human body, the normal temperature of the body is 36o C and the highest body temperature we can tolerate is 42o C. This 6o C interval is indeed very small and even working under the sun for a few hours can increase body temperature by that amount. Yet, our bodies spend a great amount of thermal energy through sweating, that is, by causing the water it contains to evaporate, which in turn causes body temperature to drop. If our bodies did not have such an automatic mechanism, working for even a few hours under the sun could be fatal.
Hydrogen bonds equip water with yet another extraordinary property, which is water's being more viscous in its liquid state than in its solid state. As a matter of fact, most substances on earth are more viscous in their solid states than in their liquid states. Contrary to other substances, however, water expands as it freezes. This is because hydrogen bonds prevent water molecules from bonding to each other too tightly, and thus many gaps are left in between them. Hydrogen bonds are broken down when water is in liquid state, which causes oxygen atoms to come closer to each other and form a more viscous structure.
This also causes ice to be lighter than water. Normally, if you melt any metal and throw in it a few solid pieces of the same metal, these pieces would sink directly to the bottom. In water, however, things are different. Icebergs weighing ten thousands of tons float on water like corks. So, what benefit can this property of water provide us?
Let us answer this question with the example of a river: When the weather is very cold, it is not the whole river, but only the surface of it that freezes. Water reaches its heaviest state at + 4o C, and as soon as it reaches this temperature, it immediately sinks to the bottom. Ice is formed on top of water as a layer. Under this layer, water continues to flow, and since + 4o C is a temperature at which living organisms can survive, life in water continues.
These unique properties which Allah has given water make life possible on the earth. In the Qur'an, Allah states the importance of this great blessing He offers man:
It is He Who sends down water from the sky. From it you drink and from it come the shrubs among which you graze your herds. And by it He makes crops grow for you and olives and dates and grapes and fruit of every kind. There is certainly a Sign in that for people who reflect. (Surat an-Nahl: 10-11)
An Interesting Property of Water
We all know that water boils at 100o C and freezes at 0o C. In fact, under normal circumstances, water should be boiling not at 100o C but at + 180o C. Why?
In the periodic table, the properties of elements in the same group vary in a progressive form from light elements towards heavy elements. This order is most evident in hydrogen compounds. The compounds of the elements sharing the same group with oxygen in the periodic table are called "hydrides". In fact, water is "oxygen hydride". Hydrides of other elements in this group have the same molecular structure as the water molecule.
The boiling points of these compounds vary in a progressive way from sulphur to heavier ones; however, the boiling point of water unexpectedly goes against this pattern. Water (oxygen hydride) boils at 80o C less than it is supposed to. Another surprising situation has to do with the freezing point of water. Again, according to the order in the periodic system, water is supposed to freeze at – 100o C. Yet, water breaks this rule and freezes at 0o C, 100o C above the temperature at which it is due. This brings to mind the question as to why no other hydride, but only water (oxygen hydride) disobeys the rules of the periodic system.
 |
| Molecules at the surface of a liquid feel a net force pulling inward. This is surface tension. It provides a cohesive force between the surface molecules, which is sufficient to prevent the legs of a ripple bug from breaking through. The high surface tension in water is vital to physiological processes.31 |
The laws of physics, the laws of chemistry, and all the other things we name as rules are just attempts at explaining the extraordinary equilibrium in the universe, and the details of creation. All research conducted in the 20th century shows more than ever that all the physical balances in the universe are tailor-made for human life. Research reveals that all the laws of physics, chemistry and biology prevalent in the universe as well as the atmosphere, sun, atoms and molecules, etc., are all arranged just as they are needed in order to support human life. Water, like the other elements mentioned above, is fit for life to such a degree as not to be comparable to any other liquid, and a major portion of the earth is filled with water in just the right amounts required for life. It is obvious that all these cannot be coincidences and that there is perfect order and design prevalent in the universe.
Allah is He Who created the heavens and the earth and sends down water from the sky and by it brings forth fruits as provision for you. He has made the ships subservient to you to run upon the sea by His command, and He has made the rivers subservient to you, and He has made the sun and moon subservient to you holding steady to their courses, and He has made the night and day subservient to you. He has given you everything you have asked Him for. If you tried to number Allah's blessings, you could never count them. Man is indeed wrongdoing, ungrateful. (Surah Ibrahim: 32-34)
The staggering physical and chemical properties of water reveal that this liquid has been created specially for human life. Allah gave life to people through water and by it has brought forth from the earth everything they need in order to live. Allah summons people to think about this subject in the Qur'an:
It is He Who sends down water from the sky from which We bring forth growth of every kind, and from that We bring forth the green shoots and from them We bring forth close-packed seeds, and from the spathes of the date palm date clusters hanging down, and gardens of grapes and olives and pomegranates, both similar and dissimilar. Look at their fruits as they bear fruit and ripen. There are Signs in that for people who believe. (Surat al-An'am: 99)
The Protective Ceiling: Ozone
The air we breathe, that is, the lower atmosphere, is in the main composed of oxygen gas. By oxygen gas, we mean O2. That is to say that the oxygen molecules in the lower atmosphere are each comprised of two atoms. However, the oxygen molecule may sometimes be comprised of three atoms (O3). In this case, this molecule is no longer called oxygen, but "ozone", because these two gases are quite different from each other.
One point needs mention here: while oxygen is formed when two oxygen atoms combine, why is a different gas called ozone formed when three oxygen atoms combine? Eventually, isn't it the oxygen atom that combines, be it two or three atoms in a molecule? Why then do two different gases emerge? Before answering these questions, it would be better to see what differentiates these gasses from each other.
Oxygen (O2) is found in the lower atmosphere and gives life to all living beings through respiration. Ozone (O3) is a poisonous gas with a very bad smell. It is found in the highest strata of the atmosphere. If we had to breathe ozone instead of oxygen, none of us would survive.
 | |
| 4) O3 | How does chlorine destroy ozone?Chlorine reacts with ozone, producing an oxygen molecule and a hypochlorite ion (OCl-) (1). The ion reacts with an oxygen atom (2) to liberate free chlorine (3), which can react with and destroy another ozone molecule.32 |
The ozone is in the upper atmosphere, because there it serves a highly vital function for life. It forms a layer approximately 20 km above the atmosphere surrounding the earth like a belt. It absorbs the ultraviolet rays emitted by the sun, preventing them from reaching the earth at full intensity. Since ultraviolet rays have very high energy, their direct contact with the earth would cause everything on the earth to burn up, never allowing life to form. For this reason, the ozone layer serves as a protective shield in the atmosphere.
In order for life to exist on the earth, all living beings must be able to breathe and be protected from harmful sunrays. The one who forms this system is Allah, Who rules over each atom, each molecule. Without Allah's permission, no power whatsoever could bring these atoms together in different proportions as oxygen and ozone gas molecules.
Molecules We Taste and Smell
The senses of taste and smell are perceptions making man's world more beautiful. The pleasure derived from these senses has been a matter of interest since ancient times and it has been discovered only recently that these are caused by molecular interactions.
"Taste" and "smell" are only perceptions that are created by different molecules in our sense organs. For instance, the smells of food, drinks, or various fruits and flowers we see around us all consist of volatile molecules. So, how does this happen?
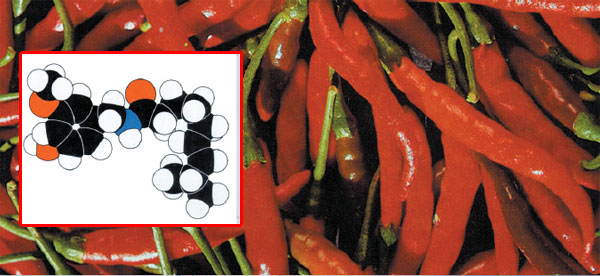 |
PIPERINEPiperine is the active component of white and black pepper (the berries of the tropical vine Piper nigrum). Black pepper is obtained by allowing the unripe fruit to ferment and then drying it. White pepper is obtained by removing the skins and pulp of the ripe berries and drying the seeds.33 |
Volatile molecules like aroma of vanilla and aroma of rose reach the receptors located on the vibrating hairs in the nasal region called the epithelium and interact with those receptors. This interaction is perceived as smell in our brains. So far, seven different types of receptors have been identified in our nasal cavity, which is lined by a smelling membrane of 2-3 cm2. Each one of these receptors corresponds to a primary odor. In the same way, there are four different types of chemical receptors in the front part of our tongue. These correspond to salty, sweet, sour and bitter tastes. Our brains perceive molecules arriving at the receptors of our sense organs as chemical signals.
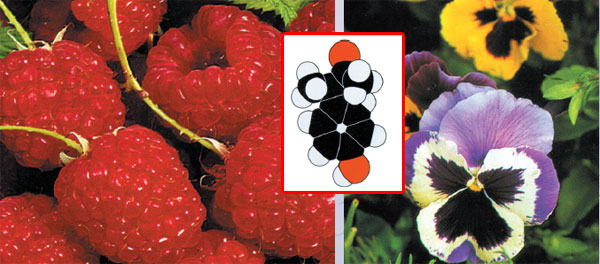 |
Para-HYDROXYPHENOL -2-BUTANONE ve IONONEThe mixture of these two molecules produces a very pleasant aroma. Butanone is the molecule chiefly responsible for the smell of ripe raspberries. The fresh new smell of the newly picked fruit is due partly to ionone, which is also responsible for the odours of sundried hay and violets. Ionone is the fragrant component of oil of violets.34 |
It has been discovered how taste and smell are perceived and how they are formed, yet scientists have so far not been able to reach agreement as to why certain substances have a strong smell while some have less and why some taste good and some bad.
 |
FURYLMETHANETHIOLThis molecule is one of those responsible for the aroma of coffee. The stimulating action of coffee is due to caffeine. The colour of roasted coffee beans seen left is largely due to the browning reaction that occurs when organic substances containing nitrogen are heated. Temporarily trapped within the beans are the molecules responsible for flavour and stimulation.35 |
Think for a minute. We could be living in a world without any flavour or odour. Since we would have no idea about the concepts of taste and fragrance, it would not even occur to us to wish to possess these perceptions. However, it is not so. Out of the brown soil with a unique smell come hundreds of types of aromatic and delicious fruits, vegetables and flowers in thousands of colours, shapes and fragrances. Why then do these atoms, which, on one hand, come together in an extraordinary way to form matter, combine, on the other hand, to produce taste and smell? Although we often take them as granted and do not remember much what a great favour they are, they pleasantly contribute to our world as products of a magnificent artistry.
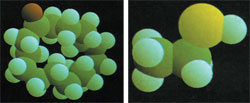 |
| The picture above belongs to an evil-smelling molecule and the one on the left to an aromatic molecule. As we can see, what distinguishes bad odour from a pleasant odour is these small differences in a microcosm which is invisible to us. |
As for other living beings, some eat only grass and some different foodstuffs. Certainly, none of these smell good, or have a great taste. Even if they do, this does not mean much for these living beings as they do not have any consciousness in the sense that human beings have. We, too, could be feeding on a single type of nutrition like them. Have you ever thought how ordinary and tasteless your life would be if you had to eat a single type of food all your life and drink only water? Therefore, taste and smell, like all other blessings, are beauties Allah, possessor of infinite grace and bounty, gave man in return for nothing. The absence of even these two senses alone would make human life quite dull. In return for all these blessings given to him, what falls to man is to try to become a person with whom Allah would be pleased. In compensation for this attitude, his Lord promises him an eternal life, which is unlimitedly furnished with blessings far superior to those that are presented to us on the earth as samples of delights to come in the hereafter. However, the recompense of a life spent ungratefully, heedlessly, and neglectful of Allah, will certainly be a just one:
And when your Lord announced: "If you are grateful, I will certainly give you increase, but if you are ungrateful, My punishment is severe." (Surah Ibrahim: 7)
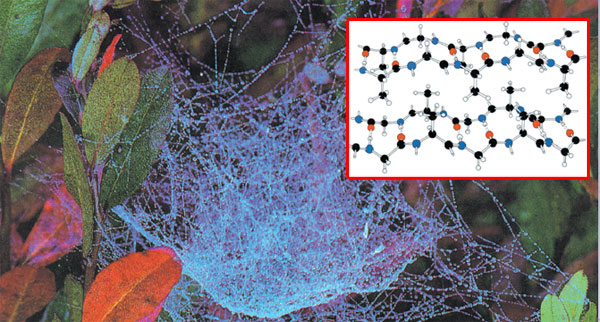 |
b-KERATINSilk, the common name of b-Keratin, is the solidified fluid excreted by a number of insects and spiders, the most valuable being the exudent of the silkworm, the caterpillar of the silk moth. It is a polypeptide made largely from glycine, alanine, and smaller amounts of other amino acids. b-Keratin molecules do not form a helix; instead they lie on top of each other to give ridged sheets of linked amino acids, with glycine appearing on only one side of the sheets. The sheets then stack one on top of the other. This planar structure is felt when you touch the smooth surface of silk.36 |
How Do We Perceive Matter?
What we have told so far has revealed that what we call matter is not an entity having a specific colour, smell and form, as we believed it to be. What we imagine to be matter, that is our own body, our room, our home, and at large, the world and the whole universe, is in reality nothing but energy. What is it then that makes everything around us visible and touchable?
The reason why we perceive the things around us as matter is the collision of electrons in the orbital shells of atoms with photons, and the atoms' attracting and repelling each other.
You are not even touching the book that you think you hold in your hand right now… In truth, the atoms of your hand are repelling the atoms of the book and you feel a sense of touch depending on the intensity of this repulsion. As we mentioned while talking about the structure of atoms, they can come close to each other at most as much as the diameter of an atom. Besides, the only atoms that can come this close are those that react with each other. Therefore, when even atoms of the same substance can by no means touch each other, it is all the more impossible for us to touch the substance we hold, squeeze or lift with our hand. In fact, if we could come as close as possible to the object in our hand, we would be involved in a chemical reaction with that object. In this case, it would be impossible for a human being or another living being to survive even for a second. The living being would immediately react with the substance on which he stepped, sat or leaned, and be transformed into something else.
The final picture that emerges in this situation is extremely remarkable: we live in a world that is 99.95 % composed of a void filled with atoms consisting almost entirely of energy.37 We actually never touch the things we say, "we touch and we hold". So, to what extent do we perceive the matter we see, hear or smell? Are these substances really as we see or hear them? Absolutely not. We had addressed this point when we talked about electrons and molecules. Remember, it is literally impossible for us to see the matter we believe to exist and see, because the phenomenon we call seeing comprises certain images formed in our brain by photons coming from the sun, or from another light source, hitting the matter, which absorbs a certain portion of the incoming light, and gives out the rest, which therefore is re-emitted from the matter and strikes our eyes. That is to say that the matter we see only consists of the information carried by photons that are reflected to our eye. So, how much of the data related to matter is conveyed to us by this information? We have no proof that the original forms of the matters existing outside are fully reflected to us.
Footnotes
24. Martin Sherwood & Christine Sulton, The Physical World, Oxford University Press, 1988, p. 81 
25. Martin Sherwood & Christine Sulton, The Physical World, Oxford University Press, 1988, p. 82
26. Martin Sherwood & Christine Sulton, The Physical World, Oxford University Press, 1988, p. 79
27. L. Vlasov, D. Trifonov, 107 Stories About Chemistry, 1977, p. 117
28. L. Vlasov, D. Trifonov, 107 Stories About Chemistry, 1977, p. 118
29. David Burnie, Life, Eyewitness Science, London: Dorling Kindersley, 1996, p.8
30. Nevil V. Sidgwick, The Chemical Elements and Their Compounds, vol.1, Oxford: Oxford University Press, 1950, p.490
31. Martin Sherwood & Christine Sulton, The Physical World, Oxford University Press, 1988, p. 30
32. Structure of Matter, The Time Inc. Book Company, 1992, p. 76
33. P.W. Atkins, Molecules, Scientific American Library, p. 115
34. P.W. Atkins, Molecules, Scientific American Library, p. 128
35. P.W. Atkins, Molecules, Scientific American Library, p. 130
36. P.W. Atkins, Molecules, Scientific American Library, p. 93
37. Taşkın Tuna, Uzayın Ötesi (Beyond Space), Boğaziçi Yayınları, 1995, p. 166