Chapter III: The Rhythm of The Atoms
If the world's finest minds can unravel only with difficulty the deeper workings of nature, how could it be supposed that those workings are merely a mindless accident, a product of blind chance?Paul Davies, Professor of Theoretical Physics 31
Scientists are in general agreement that, on the basis of calculations, the Big Bang took place about 17 billion years ago. All the matter making up the universe was created from nothingness but with the wonderful Creation that we talked about in the first two chapters. Nevertheless, the universe that emerged from the Big Bang could have been much different from the one that did emerge–ours.
 |
For example, if the values of four fundamental forces were different, the universe would have consisted of only radiation and become a tissue of light with no stars, galaxies, human beings, or anything else. Due to the extraordinary perfect balance of those four forces, "atoms"–the building-blocks of that which is called "matter"–came into being.
Scientists are also in general agreement that the first two simplest elements–hydrogen and helium–began to form during the first fourteen seconds after the Big Bang. The elements were formed as a result of a reduction in the universal entropy that was causing matter to scatter everywhere. In other words, at first the universe was just an amassing of hydrogen and helium atoms. If it had remained so, again there could have been no stars, planets, stones, soil, trees, or human beings. It would have been a lifeless universe consisting of only those two elements.
Carbon, the fundamental element of life, is a much heavier element than hydrogen and helium. How did it come into being?
Searching for an answer to this question, scientists stumbled upon one of the most surprising discoveries of this century.
The Structure of the Elements
 |
| 1. neutron |
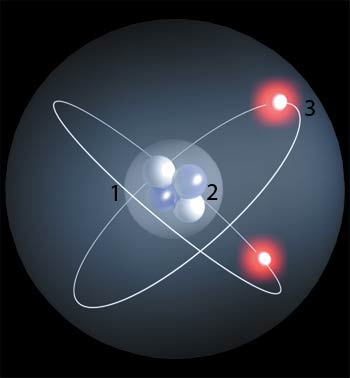
Chemistry is a science that deals with the composition, structure, and properties of substances and with the transformations that they undergo. The bedrock of modern chemistry is the periodic table of elements. First laid out by Russian chemist Dmitry Ivanovich Mendeleyev, the elements in the periodic table are arranged according to their atomic structures. Hydrogen occupies the first place in the table because it is the simplest of all the elements, consisting of only one proton in its nucleus and one electron revolving around it.
Protons are subatomic particles that carry a positive electrical charge in the nucleus of an atom. Helium, with two protons, occupies the second place in the periodic table. Carbon has six protons and oxygen has eight. All the elements differ in the number of protons that they contain.
Another particle present in the nucleus of an atom is the neutron. Unlike protons, neutrons do not carry an electrical charge: they are neutral in other words, hence their name.
The third basic particle of which atoms are composed is the electron, which has a negative electrical charge. In every atom, the number of protons and electrons is the same. Unlike protons and neutrons however, electrons are not located in the nucleus. Instead, they move around the nucleus at a very high speed that keeps the positive and negative charges of the atom apart.
The differences in atomic structure (the numbers of protons/electrons) are what make the elements different from one another.
A crucial rule of (classical) chemistry is that elements cannot be transformed into one another. Changing iron (with twenty-six protons) into silver (with eighteen) would require removing eight protons from the nucleus. But protons are bound together by the strong nuclear force and the number of protons in a nucleus can be changed only in nuclear reactions. Yet all the reactions that take place under terrestrial conditions are chemical reactions that depend on electron exchange and that do not effect the nucleus.
In the Middle Ages there was a "science" called alchemy–the forerunner of modern chemistry. Alchemists, unaware of the periodic table or the atomic structures of the elements, thought it was possible to transform one element into another. (A favorite object of pursuit, for reasons that should be apparent, was trying to turn iron into gold.) We now know that what the alchemists were trying to do is impossible under normal conditions such as exist on Earth: The temperatures and pressures required for such a transformation to take place are too enormous to achieve in any terrestrial laboratory. But it is possible if you have the right place to do it in.
And the right place, it turns out, is in the hearts of stars.
The Universe's Alchemy Labs: Red Giants
 |
| Red giants are huge stars about fifty times bigger than our Sun. Deep within these giants, an extraordinary process takes place. |
The temperature required to overcome the reluctance of nuclei to change is nearly 10 million degrees Celsius. This is why "alchemy" in the real sense takes place only in stars. In medium-sized stars like the Sun, the enormous energy being radiated is the result of hydrogen being fused into helium.
Keeping this brief review of the chemistry of elements in mind, let us return to the immediate aftermath of the Big Bang. We mentioned that only helium and hydrogen atoms existed in the universe after the Big Bang. Astronomers believe that solar-type stars (of which the Sun is one) are formed as a result of nebulae (clouds) of hydrogen and helium gas being compressed until the hydrogen-to-helium thermonuclear reaction gets started. So now we have stars. But our universe is still lifeless. For life, heavier elements–oxygen and carbon specifically–are required. There needs to be another process whereby hydrogen and helium can be converted into still other elements.
The "manufacturing-plants" of these heavy elements it turns out are the red giants–a class of stars that are fifty times bigger than the Sun.
Red giants are much hotter than solar-type stars and this characteristic enables them to do something other stars cannot: They convert helium into carbon. Nevertheless, even for a red giant this is not easy. As the astronomer Greenstein says: "Even now, when the answer (as to how they do it) is well in hand, the method they employ seems astonishing.""32
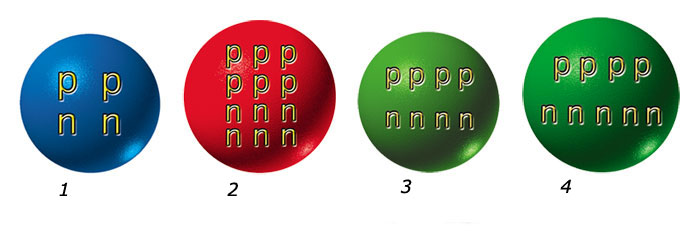
Helium's atomic number is 2: that is, it has two protons in its nucleus. Carbon's atomic number is 6. In the fantastically high temperatures of red giants, three helium atoms are fused into a carbon atom. This is the "alchemy" that supplied the universe with its heavier elements after the Big Bang.
But as we said: it's not easy. It's nearly impossible to persuade two helium atoms to join together and quite impossible for three. So how do the six protons needed for carbon get together?
It's a two-step process. First, two helium atoms are fused into an intermediary element with four protons and four neutrons. Next, a third helium is added to this intermediary element to make a carbon atom with six protons and six neutrons.
The intermediary element is beryllium. Beryllium occurs naturally on Earth but the beryllium that occurs in red giants is different in a crucially important way: It consists of four protons and four neutrons, whereas terrestrial beryllium has five neutrons. "Red-giant beryllium" is a slightly different version. It's what's called an "isotope" in chemistry.
Now comes the real surprise. The "red-giant" isotope beryllium turns out to be incredibly unstable. Scientists have studied this isotope for years and discovered that once it has formed, it breaks down again in just 0.000000000000001 second.
How is this unstable beryllium isotope, which forms and disintegrates in such a short time, able to unite with a helium atom to become a carbon atom? It is like trying to lay a third brick on two other bricks that shoot away from each other in 0.000000000000001 second if they chance to come atop one another, and form a construction in this way. How does this process take place in red giants? Physicists scratched their heads over this puzzle for decades without coming up with an answer. The American astrophysicist Edwin Salpeter finally discovered a clue to the mystery in the concept of "atomic resonance".
Resonance and Double Resonance
 |
| 1. Helium nucleus, |
Resonance is defined as the harmony of frequencies (vibrations) of two different materials.
A simple example from ordinary experience will give us an idea of what physicists mean by "atomic resonance". Imagine yourself and a child at a playground where there are swings. The child sits on the swing and you give him a push to get him started. To keep the swing moving, you have to keep pushing it from behind. But the timing of these pushes is important. Each time the swing approaches you, you have to apply the force of the push just at the right moment: when the swing is at the highest point of its motion towards you. If you push too soon, the result is a collision that disturbs the rhythmic momentum of the swing; if you push too late, the effort is wasted because the swing is already moving away from you. In other words, the frequency of your pushes must be in harmony with the frequency of the swing's approaches to you.
Physicists refer to such a "harmony of frequencies" as "resonance". The swing has a frequency: for example it reaches you every 1.7 seconds. Using your arms you push it every 1.7 seconds. Of course if you want, you can change the frequency of the swing's motion, but if you do, you have to change the frequency of the pushes as well, otherwise the swing will not swing right.33
Just as two or more moving bodies can resonate, resonance can also occur when one moving body causes motion in another. This type of resonance is often seen in musical instruments and is called "acoustic resonance". It can occur, for example, among two finely-tuned violins. If one of these violins is played in the same room as the other, the strings of the second will vibrate and produce a sound even though nobody is touching it. Because both instruments have been precisely tuned to the same frequency, a vibration in one causes a vibration in the other.34
The resonances in these two examples are simple ones and are easy to keep the track of. There are other resonances in physics that are not simple at all and in the case of atomic nuclei, the resonances can be quite complex and sensitive.
Every atomic nucleus has a natural energy level that physicists have been able to identify after lengthy study. These energy levels are quite different from one another but a few rare instances of resonance between atomic nuclei have been observed. When such resonance occurs, the motions of the nuclei are in harmony with one another like our examples of the swing and violin. The important point of this is that the resonance expedites nuclear reactions that can affect the nuclei.35
 |
| Fred Hoyle was the first to discover the amazing equilibrium of nuclear reactions taking place in red giants. Although an atheist, Hoyle admitted that this balance could not be explained by chance and that it was a deliberate arrangement. |
Investigating how carbon was made by red giants, Edwin Salpeter suggested that there must be a resonance between helium and beryllium nuclei that facilitated the reaction. This resonance, he said, made it easier for helium atoms to fuse into beryllium and this could account for the reaction in red giants. Subsequent research however failed to support this idea.
Fred Hoyle was the second astronomer to address this question. Hoyle took Salpeter's idea a step further, introducing the idea of "double resonance". Hoyle said that there had to be two resonances: one that caused two heliums to fuse into beryllium and one that caused the third helium atom join this unstable formation. Nobody believed Hoyle. The idea of such a precise resonance occurring once was hard enough to accept; that it should occur twice was unthinkable. Hoyle pursued his research for years and in the end he proved that his idea was right: there really was a double resonance taking place in the red giants. At the exact moment two helium atoms resonated in union, a beryllium atom appeared in the 0.000000000000001 second needed to produce carbon. George Greenstein describes why this double resonance is indeed an extraordinary mechanism:
There are three quite separate structures in this story–helium, beryllium, and carbon–and two quite separate resonances. It is hard to see why these nuclei should work together so smoothly…Other nuclear reactions do not proceed by such a remarkable chain of lucky breaks…It is like discovering deep and complex resonances between a car, a bicycle, and a truck. Why should such disparate structures mesh together so perfectly? Upon this our existence, and that of every life form in the universe, depends.36
In the years that followed it was discovered that other elements like oxygen are also formed as a result of such amazing resonances. A zealous materialist, Fred Hoyle's discovery of these "extraordinary transactions" forced him to admit in his book Galaxies, Nuclei and Quasars, that such double resonances had to be the result of Creation and not coincidence. 37In another article he wrote:
If you wanted to produce carbon and oxygen in roughly equal quantities by stellar nucleosynthesis, these are the two levels you would have to fix, and your fixing would have to be just about where these levels are actually found to be 38
And Hoyle continues saying that commonsense interpretation of the above mentioned facts suggests that a super Intellect has created physics, as well as chemistry and biology, and that there are no blind forces worth speaking about in nature. He adds that "the numbers one calculates from the facts seem to me so overwhelming as to put this conclusion almost beyond question."
Hoyle declared that the inescapable conclusion of this plain truth should not go unnoticed by other scientists.
I do not believe that any scientist who examined the evidence would fail to draw the inference that the laws of nuclear physics have been deliberately designed with regard to the consequences they produce inside the stars.39
This plain truth was expressed in the Qur'an 1,400 years ago. Allah indicates the harmony in Creation of the heavens in the verse: Do you not see how Allah created seven heavens in harmony… (Surah Nuh: 15)
A Lesser Alchemy Lab: The Sun
 |
| 1. Hydrogen fuel, |
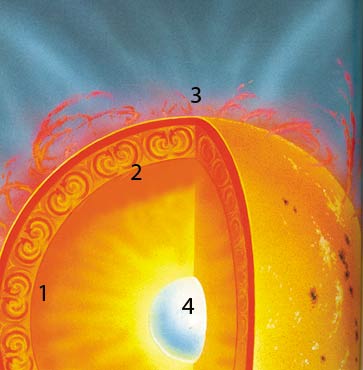
| The Sun is a giant nuclear reactor that constantly transforms atoms of hydrogen into helium and produces heat in the process. What is crucial to this process however is the incredible precision with which these reactions are balanced within the Sun. The slightest change in any of the forces governing these reactions would result in their failure or in a catastrophic runaway explosion. |
The conversion of helium into carbon described above is the alchemy of red giants. In smaller stars like our Sun, a simpler sort of alchemy takes place. The Sun converts hydrogen into helium and this reaction is the source of its energy.
This reaction is no less essential for us to exist than are the reactions in the red giants. Moreover, the Sun's nuclear reaction is also specially created, just like the one in red giants.
Hydrogen, the input element for this reaction, is the simplest element in the universe for its nucleus consists of a single proton. In a helium nucleus, there are two protons and two neutrons. The process taking place in the Sun is the fusion of four hydrogen atoms into one helium atom.
An enormous amount of energy is released during this process. Nearly all the thermal and light energy reaching Earth is the result of this solar nuclear reaction.
Like the reactions taking place in red giants, this solar nuclear reaction turns out to involve a number of unexpected aspects without which it could not take place. You can't simply jam four hydrogen atoms together and turn them into helium. To make this happen, a two-step process is required, paralleling the one taking place in red giants. In the first step, two hydrogen atoms combine to form an intermediary nucleus called deuteron consisting of one proton and one neutron.
What force could be great enough to produce a deuteron by jamming two nuclei together? This force is the "strong nuclear force", one of the four fundamental forces of the universe mentioned in the previous section. This is the most powerful physical force in the universe and is billions of billions of billions of billions times stronger than the gravitational force. Nothing but this force could unite two nuclei like this.
Now the really curious thing about all this is that research shows that, strong as it is, the strong nuclear force is just barely strong enough to do what it does. If it were even slightly weaker than it is, it would not be able to unite the two nuclei. Instead, two protons nearing each other would repel each other immediately and the reaction in the Sun fizzle out before it ever began. In other words, the Sun would not exist as an energy-radiating star. Concerning this, George Greenstein says: "Had the strong force had been only slightly less strong, the light of the world would have never been lit."40
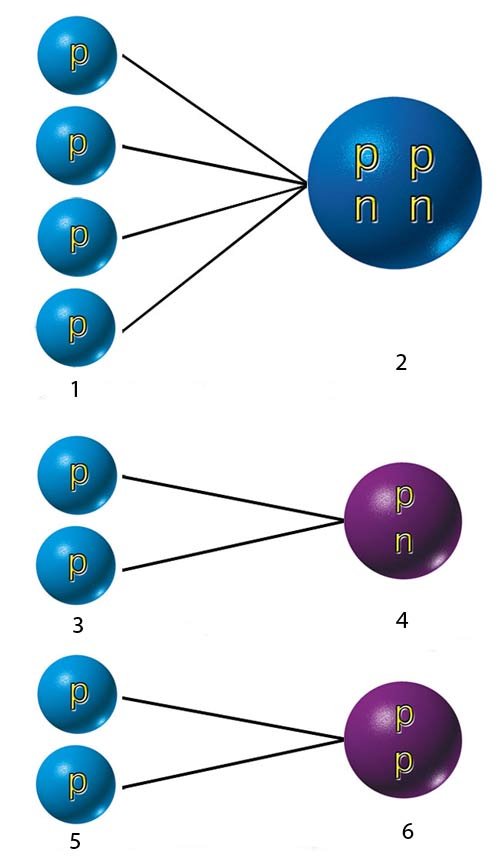 |
| 1. Single-proton hydrogen nuclei, |
THE CRITICAL REACTION IN THE SUN |
| 1) Above: Four hydrogen atoms in the Sun join together to form a single helium atom. |
What, on the other hand, if the strong nuclear force were stronger? To answer that, we first have to look at the process of converting two hydrogen atoms into a deuteron in a little more detail. First, one of the protons is stripped of its electrical charge and becomes a neutron. This neutron forms a deuteron by uniting with a proton. The force causing this unification is the "strong nuclear force"; the force that converts a proton into a neutron on the other hand is a different one and is called the "weak nuclear force". It is weak only by comparison however and it takes about ten minutes to make the conversion. At the atomic level, this is an immensely long time and it has the effect of slowing down the rate at which the reaction in the Sun takes place.
Let us now return to our question: What would happen if the strong nuclear force were stronger? The answer is that the reaction in the Sun would be changed dramatically because the weak nuclear force would be eliminated from the reaction.
If the strong nuclear force were any stronger than it is, it would be able to fuse two protons to one another immediately and without having to wait ten minutes for a proton to be converted into a neutron. As a result of this reaction, there would be one nucleus with two protons instead of a deuteron. Scientists call such a nucleus a "di-proton". It is a theoretical particle however insofar as it has never been observed to occur naturally. But if the strong nuclear force were much stronger than it is, then there would be real di-protons in the Sun. So what? Well by getting rid of the proton-to-neutron conversion, we would be eliminating the "throttle" that keeps the Sun's "engine" running as slowly as it does. George Greenstein explains what the result of that would be:
The Sun would change because the first stage in the formation of helium would no longer be the formation of the deuteron. It would be the formation of the di-proton. And this reaction would not involve the transformation of a proton into a neutron at all. The role of the weak force would be eliminated, and only the strong force would be involved…and as a result the Sun's fuel would suddenly become very good indeed. It would become so powerful, so ferociously reactive, that the Sun and every other star like it would instantaneously explode.41
The explosion of the Sun would cause the world and everything on it to burst into flames, burning our blue planet to a crisp in a few seconds. Because the strong nuclear force is precisely fine-tuned to be neither too strong nor too weak, the Sun's nuclear reaction is slowed down and the star has been able to radiate light and energy for billions of years. This precise tuning is what makes it possible for mankind to live. If there were even the slightest deviation in this arrangement, the stars (including our Sun) would not exist or if they did, they would explode in a short time.
In other words the structure of the Sun is neither accidental nor unintentional. Quite the contrary: Allah has created the Sun for people to live, as expressed in the verse:
The Sun and the Moon follow courses (exactly) computed. (Surat ar-Rahman: 5)
Protons and Electrons
So far we have been examining matters concerned with forces that affect atomic nuclei. There is another important equilibrium in the atom that we must consider: the balance between its nucleus and electrons.
Put in its simplest terms, electrons revolve around the nucleus. The reason for this is electrical charge. Electrons have a negative charge and protons have a positive charge. Opposite charges attract, so an atom's electrons are drawn towards the nucleus. But the electrons are also moving at an enormous speed which would, under normal conditions, cause them to shoot away from the nucleus. These two forces (attraction and motion away) are balanced so that the electrons move in orbits around the nucleus.
Atoms are also balanced in terms of their electric charges: the number of orbiting electrons is the same as the number of protons in the nucleus. (For example, oxygen has eight protons and eight electrons.) In this way the electrical force of an atom is balanced and the atom is electrically neutral.
So far, so much basic chemistry. However there is a point in this seemingly simple structure that is overlooked by many. A proton is much bigger than an electron in terms of both size and weight. If an electron were the size of a walnut, a proton would be about the size of a man. Physically, they are quite dissimilar.
But their electrical charges are the same size!
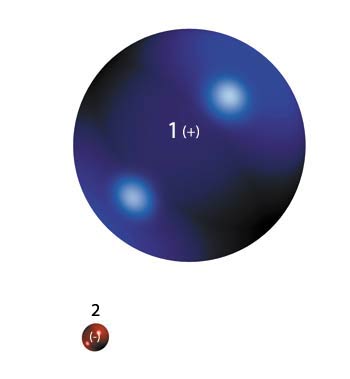 | |
| 1. Proton, | 2. Electron |
| Both the mass and the volume of a proton are incomparably larger than those of an electron but, | |
Although their electrical charges are opposite (electrons negative, protons positive) they are also equal. There is no obvious reason why this should be so. Conceivably (and "logically") an electron ought to carry a much smaller charge because it is so much smaller.
But if that were true, then what would happen?
What would happen is that every atom in the universe would be positively charged instead of being electrically neutral. And because like charges repel, every atom in the universe would try and repel every other atom. Matter as we know it could not exist.
What would happen if it suddenly became true now? What would happen if every atom were to start repelling every other?
Quite extraordinary things would happen. Let us begin with the changes that would occur in your body. The moment this change occurred, your hands and your arms holding this book would shatter at once. And not just your hands and arms but also your body, your legs, your eyes, your teeth–every part of your body would explode in a split second.
The room you sit in and the world around you would explode in a moment. All the seas, mountains, the planets in the solar system, and all the stars and galaxies in the universe would shatter into atomic dust. And there would never again be anything in the universe to observe. The universe would become a mass of disorganized atoms pushing each other around.
By how much would the sizes of the electrical charges of protons and electrons have to differ in order for this dreadful thing to happen? One percent? A tenth of one percent? George Greenstein addresses this question in The Symbiotic Universe:
Small things like stones, people, and the like would fly apart if the two charges differed by as little as one part in 100 billion. Larger structures like the Earth and the Sun require for their existence a yet more perfect balance of one part in a billion billion.42
Here is yet another precisely-tuned equilibrium that proves that the universe is created for a particular purpose. As John D. Barrow and Frank J. Tipler maintain in their book "The Anthropic Cosmological Principle", "there is a grand design in the Universe that favours the development of intelligent life."43
Of course every Creation proves the existence of a will that made it. That is Almighty Allah, Lord of all the worlds, the Power Who created the universe from nothingness, and fashioned it as He willed. As stated in the Qur'an, "He built the heaven, He raised its vault high and made it level." (Surat an-Nazi'at: 27-28)
By means of the extraordinary balances that we have seen in this chapter, matter is able to remain stable and this stability is evidence of the perfection of Allah's Creation as revealed in the Qur'an:
Among His signs is that heaven and earth hold firm by His command. (Surat ar-Rum: 25)
Footnotes
31. Paul Davies, Superforce, New York: Simon and Schuster, 1984, p. 235-236 ![]()
32. George Greenstein, The Symbiotic Universe, p. 38 ![]()
33. Grolier Multimedia Encyclopedia, 1995 ![]()
34. Grolier Multimedia Encyclopedia, 1995 ![]()
35. The resonance mentioned here occurs as follows: when two atom nuclei fuse, the new emerging nucleus both takes on the total of the massive energy of the two nuclei forming it and their kinetic energy. This new nucleus works to reach a particular energy level within the atom's natural energy ladder. However, this is only possible if the total energy it receives corresponds to this level of energy. If it fails to correspond, then the new nucleus decomposes at once. For the new nucleus to attain stability, the accumulated energy in its body and the level of natural energy it forms should be equal to each other. When this equality is attained the "resonance" occurs. However this resonance is a highly rare harmony with a very low probability to be achieved. ![]()
36. George Greenstein, The Symbiotic Universe, p. 43-44 ![]()
37. Paul Davies. The Final Three Minutes, New York: BasicBooks, 1994, p. 49-50 (Quoted from Hoyle) ![]()
38. Fred Hoyle, "The Universe:Past and Present Reflections", Engineering and Science, November 1981, pp. 8-12 ![]()
39. Fred Hoyle, Religion and the Scientists, London: SCM, 1959; M. A. Corey, The Natural History of Creation, Maryland: University Press of America, 1995, p. 341 ![]()
40. George Greenstein, The Symbiotic Universe, p. 100 ![]()
41. George Greenstein, The Symbiotic Universe, p. 100 ![]()
42. George Greenstein, The Symbiotic Universe, p. 64-65 ![]()
43. W. Press, "A Place for Teleology?", Nature, vol. 320, 1986, p. 315 ![]()
- Introduction: The Scientific Collapse of Materialism
- Chapter I: The Creation of The Universe From Nothing-ness
- Chapter II: The Equilibrium in The Explosion
- Chapter III: The Rhythm of The Atoms
- Chapter IV: The Order in The Skies
- Chapter V: The Blue Planet
- Chapter VI: The Signs of Creation in Light
- Chapter VII: The Signs of Creation in Water
- Chapter VIII: The Specially-Created Elements of Life
- Conclusion: An Appeal To Reason
