The Miracle in the Atom
 |
Whenever you grasp a door handle, shake hands with a friend, or pat your dog, the sensations that arise in your hand are nothing more than the interaction of the electrons in the molecules comprising your hand with the electrons in the atoms comprising the door handle, your friend's hand, or the dog's fur. The strong wind that blows outside in stormy weather is actually no more than molecules that comprise the air approaching at high speed and striking the atoms that comprise you. The boiling of water in a kettle is the rapid movement of its molecules, transforming from a liquid to a gas under the effect of heat. In short, everything in the universe, great or small, consists of atoms, and what we perceive as heat or cold is the result of their swift or slow vibrations.
What makes atoms so miraculous is their extraordinarily small size and the features they possess. The diameter of the atom measures around one millionth of a millimeter (1 millimeter = 0.039 of an inch). To help you to better understand 100 million atoms placed side by side would make a line only 1 centimeter long. A single page of this book you are reading is just 1 million atoms thick.1 When you realize that atoms make up everything in the universe, without exception—the giant spiral nebulae with their millions of stars, the planets, the Earth's mountains and seas—makes the extraordinary miracle here even more apparent.
Another astonishing fact about the minute atoms is that 99.9999999% of its tiny volume actually consists of empty space! The remaining portion, of the atom—less than 0.1%—consists of protons, neutrons, electrons, which are in turn composed of various subatomic particles. The neutrons and protons are fixed at the center of the atom and make up its nucleus. Yet the volume of the nucleus is just one ten billionth of the atom's volume. In constant revolution around this nucleus are the electrons, are so small that under an electron microscope their image is no more than a cloud of dust. Their mass is just 1/1,840 of that of a proton. In order to better understand this ratio, imagine that you have divided a tiny pinpoint into 1,840 parts. The electron is vastly smaller than any of these, because the larger proton possesses a mass many millions of times smaller than anything we can see. This example illustrates just how small this micro world really is. 2
That part of the atom described as being "full" consists of these tiny particles. If it were possible to remove all the empty space in all the atoms in New York City's Empire State Building, the matter remaining would be smaller in volume than a box of sugar. Yet its weight—or as physicists call it, mass—would remain unchanged, and it would be impossible to lift this small box with even the most powerful winches. 3
 |
1-The Empire State Bulding |
If you remove all the empty space inside the atoms com pris ing the Empire State Building, its vol ume will be that of a box of sugar. However, they will lose none of their mass. Not even the most pow er ful winch es will be able to move this box. |
Why would an atom's mass remain the same when all empty space in it is removed? Because all its mass or density lies in the nucleus and the electrons that comprise it. Therefore, even though the nucleus and electrons represent less than 0.1% of the atom's volume, they still exert an extraordinary force.
An atom's diameter may be as smaller than a billionth of a centimeter (1 centimeter equals 0.4 of an inch). But the subatomic particles are hundreds of thousands of times smaller than the atom itself. Almost all the atom is empty. If an atoms' nucleus were enlarged to the size of a grain of rice, the size of the whole atom would be that of a football stadium, with the electrons as minute specks of dust flying around the outer stands. At the beginning of the 20th century, British physicist Sir Arthur Eddington dramatized this fact:
I am sitting at a table, writing this paper. However, when I describe this "real" table in the language of science as I understand it, it is "a ghost"; in fact it is made of atoms that are themselves mostly empty space..4
The physicist and psychologist Peter Russell states that in fact, the 0.0000001% in question does not represent matter as we know it:
With the development of quantum theory, physicists have found that even subatomic particles are far from solid. In fact, they are not much like matter at all—at least, nothing like matter as we know it. They can't be pinned down and measured precisely. Much of the time they seem more like waves than particles. They are like fuzzy clouds of potential existence, with no definite location. Whatever matter is, it has little, if any, substance. 5
Hans-Peter Durr, a professor of physics and head of the Max-Planck Physics Institute, clearly expresses the fact that "matter was not made from matter." 6
Therefore, even though you perceive that the matter we touch is hard in the structure of matter, there is actually nothing to give rise to this solid hardness. The atoms that comprise that matter consist of no more than empty spaces and energy waves.
The Forces That Hold The Atom Together
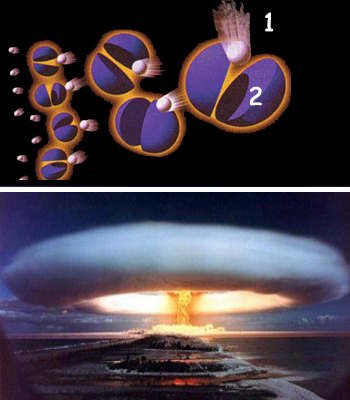 |
1. Neutron |
The reaction begins when a neutron strikes the nucleus of a plutonium atom or a uranium atom. The result ingimbal ance forces the nucleus to split, energy to be released and the two emerging neutrons to be set free to divide other nuclei. The force released by the splitting of a single atom is truly gigantic. |
How can particles too small to be seen with the naked eye be arranged in empty space to form an atom? These particles give rise to the atom with a very special creation. One of the most important features of this special creation is the basic forces that cause particles to both attract and repel each other.
These basic atomic forces act on the particles comprising the atom, in the same way that larger forces control all the more observable balances in the universe, from atmospheric pressures to the Earth's orbit. These fundamental atomic forces are known as the Strong Nuclear Force, the Weak Nuclear Force, the Force of Gravity and Electromagnetic Force.
These forces are calculated at such fine levels that the slightest change in them would lead to the extinction of life, to planets eventually colliding with one another, and the collapse of the universe itself. For example, if the force of gravity were slightly stronger or weaker, the fixed orbits of the stars would be affected:
They would either move ever closer to one another and eventually collapse into massive black holes, or move apart, eventually to drift haphazardly through the voids of space. These fundamental forces have been created at precise levels in such a way as to ensure a flawless balance in both the tiny micro world as well as across the most enormous interstellar dimensions. Each force is the product of a Divine creation, planned to fulfill its own special purpose in the universe. This belongs to Allah, Who has created everything flawlessly, from the greatest to the smallest. In a verse, Allah reveals that He possesses the knowledge of all things in the heavens and Earth, from the largest to the smallest:
… He is the Knower of the Unseen, Whom not even the weight of the smallest particle eludes, either in the heavens or in the Earth; nor is there anything smaller or larger than that which is not in a Clear Book. (Surah Saba': 3)
Of these forces, Strong Nuclear Force provides a most important equilibrium within the atom. All things being equal, under ordinary circumstances, the protons in the nucleus should repel one another and move as far apart as possible. That is because all protons are positively charged, and identical charges always repel each other—as you can demonstrate by bringing together the north poles of two separate magnets. As a result of Strong Nuclear Force, however, protons are clamped against one another, along with neutrons that bear no charge, at the center of the atom inside the atom's nucleus. In other words, Strong Nuclear Force allows the atom's nucleus to exist by holding its protons together. To better comprehend the power of this force, consider the effect of an atomic bomb.
A nuclear explosion results of when a particle—generally a neutron—is hurled to "split" the nucleus of a uranium or plutonium atom. As the nucleus comes apart, the strong force is released that formerly held the protons and neutrons. The release of incomparable energy vaporizes everything nearby, and the radioactive after-effects linger on for hundreds of years. This force concealed within an atom is imperceptible, but its power leaves any creature exposed to it utterly helpless and defenseless. The Strong Nuclear Force operating in the nucleus is finely balanced to possess the ideal values for the formation of matter, and has maintained the existence of the universe ever since it came into being. If this force were even slightly more powerful, protons and neutrons would combine with one another. Were it slightly less powerful, these particles would separate from each other, dissolving the atom and creating a subatomic "soup" of particles that would prevent the formation of any animate or inanimate entities, the Earth, the Sun or even the universe itself.
 |
The four basic for ces con trol ling all the bal an ces in the uni verse are so del i cate that even the slight est alter a - tion in their lev els might lead to the extinc tion of life. A small impair ment in a sen si tive bal ance might lead the plan ets to fall into one anoth er and turn into clouds of frag ments and for the uni verse itself to cease to exist. |
Another force that serves to maintain atoms' balanced structure is the Weak Nuclear Force, which is of particular importance in atoms with large numbers of protons and neutrons. This force prevents any neutron in the nucleus from acquiring a positive charge and turning into a proton in the atom, and thus stops the atom splitting. This is a most important precaution because, as will be remembered, the splitting of the atom gives rise to a force that leads to the atom bomb. This situation, which may arise uncontrolled in certain atoms, represents a grave danger, but it is eliminated by the effect of Weak Nuclear Force.
The Strong and Weak Nuclear Forces do not affect the atom's electron as they do its protons and neutrons: Electrons are not affected in the same way as the other particles because they are so much smaller, are in constant motion and possess little mass. Electrons revolve without departing from their orbits around the nucleus because of the Electromagnetic Force's effect on them. Due to its negative electrical charge, an electron revolves constantly around the positively charged nucleus. The centrifugal force that arises during the rotation of the electron on its orbit is exactly balanced by Electromagnetic Force, and the electron thus remains in its orbit. The delicate level of the Electromagnetic Force keeps electrons from being drawn into to the nucleus or totally departing from it. That is how the structure of the atom arises.
The Miracle in The Atom | ||
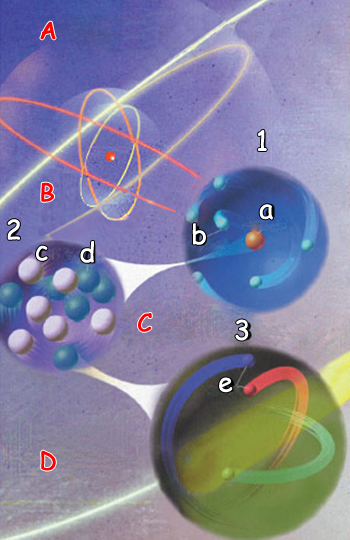 | 1. Atom : 2. Nucleus: 3. Proton: | a) Nukleus |
A. An ordinary substance consists of atoms that combine through electro magnetism to give rise to the molecules that con stitute solids, gasses and liquids. B. Atoms consist of adense nucleus surrounded byacloud of electrons. Electromagnetic for ces keep the electrons in orbit around the nucleus. C. The nucleus consists of protons and neu trons, which are bound together by the Strong Nuclear Force. D. Protons and neutrons each consist of three quarks. These are held togeth er with the Strong Nuclear Force. | ||
Before moving on to electrons, of great importance in the formation of molecules, let us briefly recall on the details of the atom's structure. So far, the information you have read in summary form is the same as what you can find in any physics text. However, textbooks of that kind are not likely to emphasize the miraculously perfect structure of the atom and its thought-provoking aspects. Inanimate particles only a millionth of a millimeter (1 millimeter equals 0.04 of an inch) in size come together flawlessly to form life and non-living substances, just as they form the billions of stars, rivers, the sky, mountains, flowers, human beings and the seas. How atoms impart an order to all this creation is little discussed.
Another fact is generally seldom mentioned: The strengths of the universe's four fundamental forces are very different from one another, which differences are very delicately balanced. For example, the Strong Nuclear Force is around a billion, billion, billion, billion, billion times stronger than the force of gravity. The difference between the Strong and Electromagnetic Forces is greater than a million times million.
If these values were different, what would happen?
 |
Under the effect of the forces that affect their intense energy, electrons constantly spin around them selves and in an orbit around the nucleus. The spin electrons perform as a result of the energy they possess is one of the main causes of the equilibrium in the universe. |
The weak and strong nuclear forces, electromagnetic forces and gravitational force must all be in their exact critical values in proportion to each other, in order for the galaxies, stars and for all the living things to exist at all.
If not, protons would not stay together in the atomic nucleus. Electrons would disperse, and a single atom could not exist. The entire universe would consist of radiation and random particles, with no stars, planets or human beings.
If the gravitational forces were any more powerful, entire galaxies would become collapsed into black holes instead of maintaining themselves by centrifugal force. Were it less powerful, stars would not be able to form the heavier elements necessary for life on planets. For instance, if the Strong Nuclear Force of the atoms constituting your body at this moment were to weaken a little—a deviation from the actual value by only thousandth—, your body would be obliterated instantly.
Yet by means of the sensitive balance of the four fundamental forces, the atoms that constitute your body and the entire universe remain stable. This sensitivity has astonished scientists. As the famous astrophysicist Paul Davies, comments, [with] a slightly different set of numbers, the world would be a very different place. Probably we would not be here to see it. . . . And when one goes on to study cosmology incredulity mounts. Recent discoveries about the primeval cosmos oblige us to accept that the expanding universe has been set up in its motion with a cooperation of astonishing precision. 7
That the universe is arranged with an astonishing sensitivity means that it was created.
As you already saw, scientists refer to the physical forces in the universe as the "four basic forces," yet their definitions fail to account for why such forces exist and why they are so exquisitely balanced. If we reach beyond these definitions, we soon realize that Almighty Lord keeps the universe regulated at every moment.
The discovery made by modern physics is in fact nothing more than a secret revealed by Allah in the Qur'an 1,400 years ago:
Allah keeps a firm hold on the heavens and Earth, preventing them from vanishing away. And if they vanished no one could then keep hold of them. Certainly He is Most Forbearing, Ever-Forgiving. (Surah Fatir: 41)
For anyone who employs reason and conscience, that an atom consisting of more than 99.9999999% empty space possesses such vitally important properties is yet another miracle of Allah's creation. Our Lord reveals that there are proofs for believers in His creations:
In the alternation of night and day and what Allah has created in the heavens and the Earth. There are Signs for people who guard against evil. (Surah Yunus: 6)
The most important of the flawless atoms' many attributes is that they combine to create molecules. In the formation of molecules, electrons play the crucial role. Before moving on to this subject let us examine electrons.
Electrons: The Atom's Outermost Shell
Without taking into account even smaller particles only recently discovered, electrons are the smallest of the atom's basic building blocks—about 1/2,000th the size of protons and neutrons.8 With their intense energy, electrons follow a specific orbit around the nucleus. As a result of the intense energy they possess and the forces that are exerted on them, they remain in the same orbit around the nucleus and also spin on their own axes.
The energy possessed by electrons displays an impeccable equilibrium that can be dramatized by the following example. Under normal conditions, it is impossible for you to balance a plate of wide diameter on top of a long rod. Yet if you give the plate a specific rate of spin of so many rotations per second, it will remain spinning on the end of that rod. The plate will inevitably fall and break when it loses speed. Therefore, all that's necessary to attain such equilibrium is an appropriate level of energy. This is the secret underlying the fundamental balances in the universe. Momentum is what keeps the planets rotating around the Sun and the electrons around the nucleus of the atom. As a result of this angular momentum, which is regulated with the greatest sensitivity, electrons orbit around the nucleus constantly, and the rotation they perform prevents them spinning away from the nucleus.
Electrons orbit the nucleus at the truly extraordinary speed of 1,000 kilometers (621 miles) per second. 9 Yet despite this high velocity, they never collide with one another because electrons all bear a negative electrical charge and therefore repel one another. However, that fact does not answer the question of why all electrons are negatively charged. Why do identical charges repel one another? How did these particles that repel one another come to be in orbit? All these questions once again reveal the sensitive balance and creation in the atom. In fact, we are dealing with a great miracle. In some atoms, more than 100 electrons orbit the nucleus. The way that electrons are divided into up to seven orbits, revolving in levels at high speed, with no confusion ever arising and never colliding with one another, is the product of an impeccable creation.
There are up to seven different energy levels around the nucleus, which creates seven different orbits for electrons. Each electron adheres to one of these paths, depending on the energy level it possesses. The reason why electrons, which always possess the same mass and velocity, have different energy levels is a point to consider. In the system in the universe, bodies with different masses and velocities wind up in different orbits. The most familiar example of this is the planets in our own Solar System. All planets have different masses and different speeds, following different orbits. However, this arrangement does not apply to electrons. There is actually no reason for these particles—whose masses and velocities are always the same—to possess different energy levels. This is a most special state of affairs created by Allah, because it is essential that these different orbits exist in order for molecules to form. The different orbits within the atom gives rise to the molecules and compounds that make up ourselves and the entire universe. At the same time, they also give rise to colors, because one of the causes of different colors is how electrons in one orbit jump across to another' level.
Electrons which are present inside the atom move too quickly that they constitute a cloud. With the flawless order they establish to form molecules, these minute units, invisible to the naked eye, constitute the basis of all matter, animate and inanimate. As you shall see in detail in the following pages, their order is so specially created that not one single component of it could possibly have come into being by chance.
The Lord of this flawless and most superior artistry is Allah, Who reveals in one verse:
He to Whom the kingdom of the heavens and the Earth belongs. He does not have a son and He has no partner in the Kingdom. He created everything and determined it most exactly. (Surat al-Furqan: 2)
 |
Electrons revolve only in speciflc orbits. Each orbit has a specivic energy level. That energy level varies according to the distance of the orbit from the nuclcus. The closer an orbit is to the nucleus, the higher the energy. There are also ‘sub-orbits’ below cach electron’s orbits. Electrons jump from the sub orbit they are in to one with higher energy. When there is an empty space in one upper energy level the electron suddenly disappcars and then reappears in an astonishing manner in that upper energy level. However, in doing this the electron reccives significant support from outside; energy. In jumping from one orbit to a higher energy sub-orbit it has to receive an equivalent amount of energy to that between the two energy levels from the outside. The electron cannot leap to this orbit until it achieves the energy level required by the upper energy level. The energy the electron recelves from the outside is the ‘’photon.’’ The color of a body is a combination of the light rays (photons) reaching the eye by being reflected from that object. If a body illuminated with White ilght appears ‘’red,’’ that means it absorbs a great part of the solar light and only reflects the red light. The elctron absorbs the photon, the light particlc that possesses the level of energy between the two sub-orbits. The part that is not absorbed is reflected as colors. |
Footnotes
1. Phil Roxbee Cox – Max Personage, Atom ve Molekül (Atom and Molecule), Tubitak Popular Science Books, Nurol Printing 1999, p. 6
4. http://216.239.37.100/search?q=cache: 92d1mfJmodkC:www.transpersonalweb.com/wolf.shtml+%22Arthur+Eddington%22%2Batom%2Bghost&hl=en&ie=UTF-8
5. Peter Russell, From Science to God, The Mystery of Consciousness and the Meaning of Light, Part 4, Illusions of Reality, http://www.peterussell.com/SG/ch4.html
7. Paul Davies, The Accidental Universe, Cambridge: Cambridge University Press, 1982, Foreword, p.VII.
8. http://findarticles.com/p/articles/mi_gx5226/is_2002/ai_n19143697
9. http://www.madsci.org/archives/ nov2000 / 974298400.Ph.r.html
