 |
The book you are holding in your hand, your hand itself and your fingernails, your television and furniture, the chair you are sitting on, the flooring beneath it, the lamp that you read by, and the water you drink—all these are substances with entirely different properties. Since they are all made up of atoms, how is it that they can possess such totally different features and appearances? The answer lies in molecules. Combinations of atoms of the roughly 109 or so different elements of in existence, in different numbers and forms, give rise to this marvelous variety.
The variety occasioned by just 109 types of atoms forming various compounds is truly extraordinary. Every substance that forms has one or more different uses, and many of them are of vital importance for life. Consider: How many different combinations can you make with 109 components? Many, but in addition, can you ensure that all of them are functional? The number you can give is of course far less. Yet through an astonishing creation, these 109 different atoms give rise not only to an infinite variety of compounds, but also to such sensations as taste, smell, color, hardness, softness, viscosity and volatility. Not only does this magnificent variety provide countless beauties and artistry, it is also necessary for organisms to survive. For example, the fact that water can assume three states—vapor, liquid, and solid—constitute one of the fundamental prerequisites for life on Earth. (This point will be examined in greater detail later.)
How can these 109 atoms produce literally billions of different types of molecules? Here the importance of electrons becomes apparent. In order for a molecule to form, electrons are either transmitted from one atom to another, or are used in common by two atoms. In this way, a molecule consisting of at least two atoms emerges. This process is of course far too complex to be fully explained in a single paragraph. The two atoms' exchange of electrons is known as a chemical bond. Yet there is actually no "bond" at all, just one electron passing back and forth between two atoms. What binds the atoms together is the journey that electron makes from one atom to the other. The form of these chemical bonds—essentially, electron sharing—and the nature and the numbers of the atoms that combine together, determines the structure and nature of the molecule. In order to clarify the subject, let's first examine the chemical bonds that permit molecules to form.
A freely moving atom is attracted or repulsed by other atoms around it. Under this effect, two atoms may approach and attach to one another, become re-arranged to achieve a stable structure. This results in the atoms surrendering their own distinctive properties and coming into possession of new features together, and forming a new substance with entirely different characteristics. For example, two hydrogen atoms and an oxygen atom that join together give rise to a new structure—a stable water molecule.
If the newly emerged compound were not stable, it would soon dissolve. By analogy, a new organ transplanted into a patient's body during a transplant operation will impair the stable structure of that body unless it can adapt itself. In a similar way, atoms that combine together must produce a stable compound by adapting to one another.
Electrons have vitally important ways of bonding in order for the resulting molecules to remain stable, and every atom employs the bonding form appropriate to it. Let us now examine these bonds.
 |
The skysctapers in large cities, your home, the table in your kitchen, the fruit you eat, the water you drink - these are all different materials with different properties. In various amounts and configurations, the combination of the 109 elements found in nature gives rise to the astonishing variety on earth. Just as these 109 elements give rise to different substances, they also provide such details as smell, taste, color, hardness, softness, viscosity and lightness. How many different substanes can you make by combining 109 separate elements? The number will of course, be large but still a finite one. Such magnificent variety from such limited raw materials doubtless displays the great artistry of Allah. |
 |
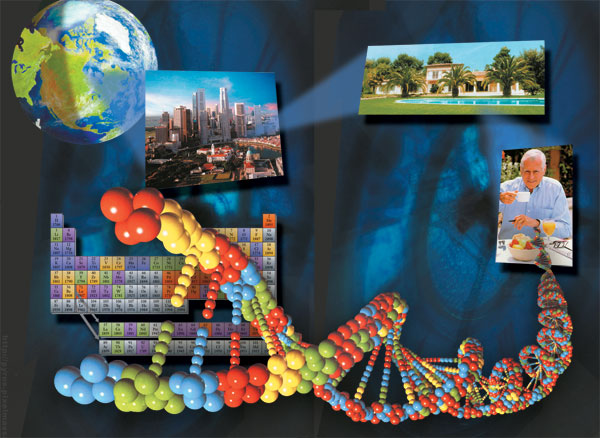
Atoms achieve a sta ble struc ture only when the num ber of elec trons in their outer shell reach es 8. The neon is a very sta ble atom with eight elec trons in its out - er most orbit.. |
The electron exchange among atoms is analogous to partners pooling their capital to start a new business. If one of the parties lacks sufficient funds to open a new plant, that person will declare himself a partner and borrow the needed amount of capital from another partner. Thus a lasting business agreement is arrived at. When the available capital grows, the number of eventual partners may rise.
The exchange among atoms may be compared to this. We have already mentioned electrons' orbits. The number of electrons in an atom's outermost orbit always is eight. If atoms have fewer electrons than eight, they need to establish partnerships in which they share electrons, and in this way, molecules can possess a stable structure. To form compounds (i.e, partnerships), atoms must either donate electrons from their outermost orbit around the nucleus to another atom, or else borrow one or more electrons from that atom. Following this exchange, the electron-donating atom will have a positive charge and the receiving atom a negative one. Since opposites attract, these two atoms will not split apart from one another. In this way is formed what's known as an ionic bond, and a molecule results.
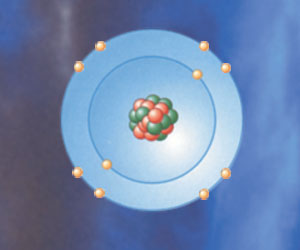
For the transfer of a large number of electrons in exchanges between atoms, a considerable amount of energy is necessary. For that reason, the most economical partnership is determined. For example, a chlorine atom has seven electrons in its outer orbit. Instead of lending seven electrons to another atom, it will be enough to receive one electron from another atom in order to complete its "capital." The most appropriate atom to donate an electron is sodium, because of the single extra electron it possesses in its outer shell. When sodium lending its one spare electron to the chlorine atom, the sodium chloride molecule is formed—and the result of this partnership is the salt you use in cooking and eating. Ordinary table salt is nothing more than a single electron exchanged between these two atoms. One important point to remember is that pure sodium is actually explosive, and that pure chlorine, a gas, is poisonous. Yet as a result of flawless planning, the mixture of explosive and poisonous atoms emerges as a substance that meets our culinary needs.
 | |
Na | Cl |
The sodium atom donates its single outer most electron to the chlorine atom with its seven outer most electrons in order for both to attain a stable state. The molecule formed by these two atoms, bound together with anionic bond, is sodium chloride, the table salt you use in every day life. But by itself, sodium is explo sive and chlorine is poisonous. | |
The business partnership we used as an analogy is a conscious agreement between two rational human beings, performed after certain analyses, with profit and loss being taken into account. A human partnership may give rise to various problems, and today's analyses may no longer be valid tomorrow. Yet the exchange that takes place between molecules is sound and trouble-free. Every atom behaves as if it knows that it should have eight electrons in its outermost orbit. So far, no molecular partnership has ever taken place with seven electrons, or with nine. In addition to calculating the number of electrons in their respective outer orbits, atoms must also determine whether it will be more profitable to donate or receive electrons.
Can this consciousness belong to the atom itself? Does the atom plan its bonding or become aware of it? Such an idea is of course impossible. That conscious planning belongs to Allah, Who creates atoms and inspires in them their systematic, inerrant behavior.
The outer electron shell that enables atoms to construct bonds with one another and which permits chemical reactions is a miracle by itself. If atoms had no tendency to limit the number of electrons in their outermost orbits, then no molecules or compounds could form anywhere in the universe, and life would therefore not be possible. So why do atoms have such a tendency? Scientists have no answer to that question!
The only explanation for the way that atoms are structured to form compounds ideally suited to life, is creation. The atomic structure has been determined in such a way as to make possible the formation of chemical bonds, and Allah has created the laws of nature that will permit this perfect order. This again reminds us that He has created the entire universe, and of the purpose and great wisdom behind His creation. In one verse, Allah states:
This is Allah's creation. Show me then what those besides Him have created! The wrongdoers are clearly misguided. (Surah Luqman: 11)
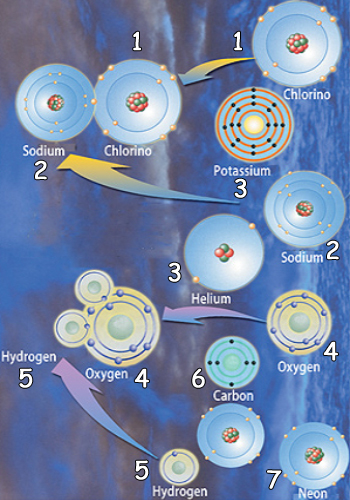 | |||
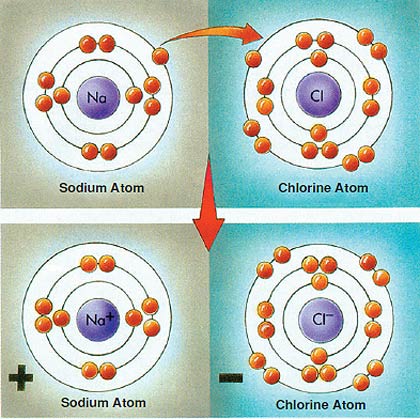
1. Chlorino | 3. Potassium | 5. Hydrogen | 7. Neon |
The proportions at which sodium combines with chlorine, and at which hydrogen binds widt oxygen, have not been determined at random.It is Allah.Who determines all such proportions and creates them in immaculate harmony. | |||
Atoms may sometimes lack enough electrons to donate to one another. Or instead of giving each other electrons, atoms may prefer another form of bonding. At such times they share the requisite electrons between them, literally like two islands joined together by a bridge. In this analogy, electrons constitute this connective bridge, which is known as a covalent bond between atoms. Many important molecules on Earth are the result of such bonds.
To help you understand these atomic bonds more clearly, take the example of the hydrogen atom, which possesses only a single electron. This exceedingly simple atom tries to double its single electron in order to achieve greater stability. As you have already seen, there needs to be a specific number of electrons—eight—to be circling in an atom's outermost orbit. The only exception to this rule is in an atom's first orbit, where the ideal number of electrons is two. Therefore, it is sufficient for a hydrogen atom, with its single electron in a single orbit, to obtain only one more electron to attain stability. To do so, hydrogen establishes bonds with various atoms. The hydrogen gas present in the atmosphere is nothing else than two hydrogen atoms joined by a covalent bond.
Similarly,
an oxygen atom has six electrons in its outer orbit. In order to become stable, it needs to raise that number to eight. It therefore seeks two hydrogen atoms—each of which possesses a single electron—with which it can establish a covalent bond. .
These calculations for oxygen and hydrogen are not randomly determined. It's no coincidence that oxygen has six electrons and two hydrogen atoms can make up this deficit. By means of the atoms' mutual harmony, water—the most essential substance for life—is created. Allah determines these proportions and creates stable, harmonious atoms and water. This is openly revealed in the following verse:
We send forth the pollinating winds and send down water from the sky and give it to you to drink. And it is not you who keep its stores. (Surat al-Hijr: 22)
DNA Structure | |
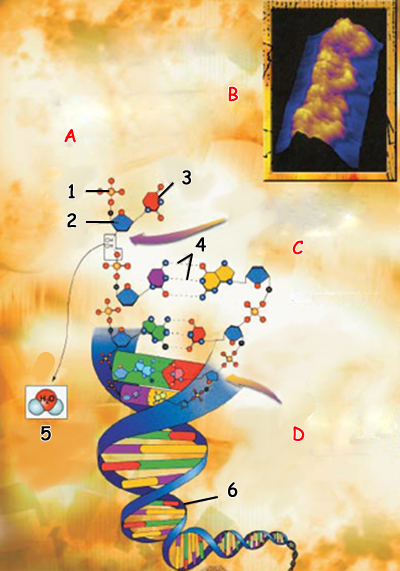 | 1. Phosphate group |
A. The molecules comprising DNA are attached with a hydrogen bond. The mirac u lous func tions that the DNA mol e cule performs result to a large extent from the flexibility of these hydrogen bonds. B. (Side) Image of DNA dou ble helix taken with a col ored micrograph (STM) C. Nucleotides attach to polymers viacova lent bonds by way of sugar and phosphate groups. A water molecule is released during this reaction. D. The covalently bonded polymerand sugar groups form the back bone of DNA. Their nitrogen bases extendinwards. Here, a great many weak hydrogen bonds link the two halves of the helix. | |
If a hydrogen atom is used in common by two atoms, this bond is known as a hydrogen bond. The two atoms in question have to be negatively charged for this to happen. Oxygen and nitrogen atoms are the best example of this. A hydrogen bond occurs when two electronegative atoms, such as nitrogen and oxygen, interact with the same hydrogen atom. Hydrogen can attach to oxygen and nitrogen atoms by means of a covalent bond. The electrons in these atoms are closer to the oxygen and nitrogen atoms compared to the hydrogen atom. The reason for this is that these other atoms have more neutrons and protons and therefore, a greater atomic weight and exert a more powerful gravitational attraction. Therefore, the electrons of the hydrogen atom and the other atom to which it will bind move away from the hydrogen atom. With negatively charged electrons moving away, the hydrogen atom's charge becomes positive, keeping it stable between the two larger, negatively charged atoms. In this way the hydrogen atom moving between the two atoms becomes such a bond, and a hydrogen bond between the two atoms is established.
 |
He emcompassed what is in their hands and has counted the exact number of everything.(Surat al-Jinn: 28) |
Hydrogen bonds are weak, meaning that a low level of energy is sufficient to break the bond. Weak bonds play a most important role in the formation of larger organic molecules, because these bonds are elastic. They impart flexibility to the substances they give rise to. During this elasticity, however, no rupture takes place between any of the bonds forming the molecule.
This distinguishing feature of hydrogen bonds is of great importance for many molecules on Earth. The clearest example is the DNA molecule: The many miraculous processes it performs in the body are to a large extent results of the hydrogen bonds the molecule possesses. In due course, you shall see this in greater detail, as well as other molecules that acquire distinguishing features by means of their hydrogen bonds.
In order for life to emerge, there are more combinations of atoms than anyone can possibly guess. There are more atoms in a visible period on this page than there are stars in our galaxy. 10 The apple you hold, the home you live in, your own body, and even the planet you live on are all composed of atoms. Yet the bonds described above are nothing more than the movements of absolutely minute electrons. It is their movements that give rise to the air you breathe, the home you live in, cats and dogs, the scents of flowers, the taste of apples, the water you drink, the enzymes in your body—in short, to everything that exists.
Can you imagine how many electrons are traveling among the millions of atoms in a single punctuation mark? The emergence of such a broad, wide-ranging universe from such a microscopic sphere, which even a powerful electron microscope shows as a blurred cloud of dust, is quite extraordinary. The emergence of something out of nothing—of weight and substance from emptiness, color from colorless, and scent from no odor are all proofs of the superior nature of Allah's creation.
It is Allah, Lord of infinite intellect, might and knowledge, sovereign over the earth and sky, Who creates all things, from subatomic particles to mountains, stars and human beings. This is revealed in this verse:
Allah, there is no god but Him, the Living, the Self-Sustaining. He is not subject to drowsiness or sleep. Everything in the heavens and the Earth belongs to Him. Who can intercede with Him except by His permission? He knows what is before them and what is behind them but they cannot grasp any of His knowledge save what He wills. His Footstool encompasses the heavens and the Earth and their preservation does not tire Him. He is the Most High, the Magnificent. (Surat al-Baqara: 255)
 |
The countless molecules in the air revolve and strike one another billions of times a second. While you are sitting quietly, alone in your room, you are actually in the middle of a molecular bombardment. And not only the molecules in the air, but molecules in your skin, table and the pen in your hand are also in a constant state of vibration. Despite this intense activity, your surroundings appear calm and in equilibrium. |
When you sit quietly in a room, with no noises around you, you imagine that nothing is moving. Yet in fact, everything around you—not just the air—is in a constant state of motion. How can that be?
Electrons, the smallest particles in the molecules that compose you and this book you are reading, constantly revolve at a velocity of 1000 kilometers (621 miles) per second. In addition, these molecules are also in constant motion. The velocity of the molecules traveling in empty space is roughly equivalent to that of a bullet leaving the muzzle of a gun—greater than 1 kilometer (0.621 miles) per second. 11
The trillions of molecules in the air collide with each other billions of times every second and continue on until they strike one another again. Thus when you imagine you are sitting all alone, perfectly still, you are actually in the midst of a molecular bombardment. This bombardment can sometimes assume the state of a violent wind, powerful enough to uproot trees and tear down buildings.
It's not only the molecules in the air that move. The molecules in your skin and the book you are holding are also in a constant state of motion. You may well wonder: How can a stone wall, one that even the most powerful winch would have difficulty lifting, be in a state of motion? But a wall really does move—but this is merely in microscopic vibration, since the molecules comprising it are compressed much closer together. Despite matter all around us being comprised of particles in a constant vibration, it always appears solid and sound. Despite its inner vibration, it never suddenly breaks up or falls apart.
 |
The molecules comprising a subtance never separate from one another for no reason. A specific temperature molecules. The temperature for the evaporation of water helps maintain the water cycle that permits the amounts of fresh water in the world to remain stable. The recycling of water is created by Allah to maintain life on Earth. |
The motion of this kind among molecules also must be balanced. The vibration referred to here is a form of motion that ensures equilibrium in solid bodies. Furthermore, except in a wind or river, molecules never move in one fixed direction alone. Were such a possibility to take place, with all molecules moving in the same direction, we would be astonished to see the dining table traveling a certain distance.12 Yet we never actually encounter such a possibility because, as a blessing from Allah, the molecules comprising a solid body cancel each other out. Thus no such irregularity of their moving in a single, fixed direction ever arises.
Molecules' ability to assume different forms under the effect of thermal energy is also the result of their motion and energy. For example, water freezes into a solid state when its molecules are staying together. When the ice warms up and becomes liquid again, the molecules slide over one another as a result of their being in more rapid motion. That is why we can stir the liquid. The final stage, steam, is when water heats up even further and its molecules move well away from each other. These molecules distance themselves from each other and can easily spread out since they are no longer restrained by surface tension. That is why you can smell meals being cooked even in another room.
Why do your hands become warmer when you rub them together? Why do two sticks smolder when you rub them swiftly together? The molecules begin moving faster. The feeling of heat in your hands is the result of the energy produced by that movement.
 |
Say:"If all the sea was ink to write dows the words of my Lord, it would run out long before the words of my Lord ran out",even if we were to bring the same amount of ink again.(Surat al-Kahf: 109) |
Though molecules are in a constant motion, generally we never perceive this. The molecules in the patterns on your tablecloth are also in motion, but you never see those patterns become impaired or deformed. Your face also consists of molecules, also in motion, but this never leads to any defects arising. Every object on Earth, even the most microscopic, is in constant motion. Yet there is no sign of this to be seen around you.
But molecules' movements are by no means haphazard. The molecules sliding over one another in liquids, moving away from one another in gasses and huddled close up against one another in solids never depart from this order. The molecules that comprise a glass never split away from one another for no reason. A specific temperature is needed in order to break the molecules up. This level has been determined with a perfect measure. For example, the temperature at which water molecules decompose is quite specific—212 Fahrenheit. Yet that same temperature does not decompose the molecules in a saucepan. That is why we are able to boil water in a saucepan. A far greater temperature would be required for the molecules in the saucepan to split up.
What would happen if this were not ensured by such a delicate and bounded equilibrium, or by the unchanging standards referred to by scientists as the laws of nature? Were there no such equilibrium, then everything on Earth would melt at the same certain temperature. For example, if everything were affected at the same temperature at which water boils, then nothing, the proteins and cells in our bodies included, could remain stable. Yet we never encounter such a danger, because everything in the universe has a determined balance and measure. The fact that water evaporates when it reaches a specific temperature makes this molecule vitally important. The water cycle on Earth is the result of this specially created system of evaporation and condensation.
Every molecule possesses a feature establishing its state at this very moment. This, of course, is a sign of the might of Allah, Who has determined a measure for all things and Who has created every measure in harmony with all others. In one verse, He reveals:
... He encompasses what is in their hands and has counted the exact number of everything. (Surat al-Jinn: 28)
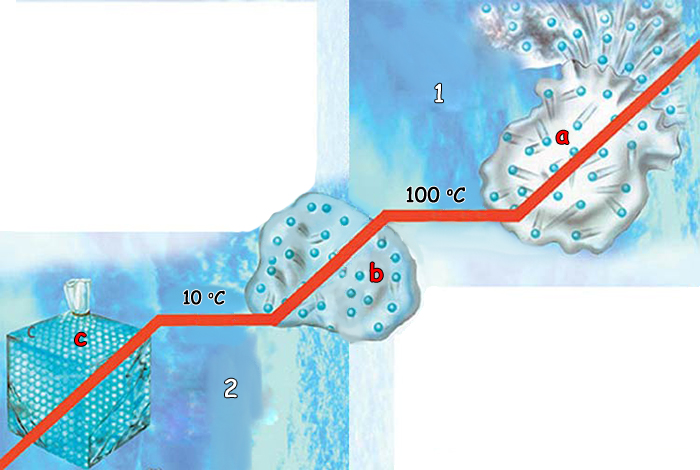 |
a. Solid |
1. When heated up, water attains a gaseous state and the molecules separate from one another even further and begin moving away from one another in the air. The temperature that causes water to boilis a special value determined by Allah. 2. Water assumes a solid form when its molecules are closest to one another. When heated, it turns into a liquid and the molecules slip over one another due to their increased motion. |