Reptile Lung
There are thousands of bird species on the earth. Every one of them possesses distinct features. For example, falcons have acute vision, wide wings and sharp talons, while hummingbirds, with their long beaks, suck the nectar of flowers.
Others migrate over long distances to very specific places in the world. But the most important feature distinguishing birds from other animals is flight. Most birds have the ability to fly.
How did birds come into existence? The theory of evolution tries to provide an answer with a long scenario. According to this story, reptiles are the ancestors of birds. Approximately 150-200 million years ago, birds evolved from their reptile ancestors. The first birds had very poor flying skills. Yet, during the evolution process, feathers replaced the thick skins of these ancient birds, which were originally covered with scales. Their front legs were also completely covered by feathers, and changed into wings. As a result of gradual evolution, some reptiles adapted themselves to flight, and thus became the birds of today.
This scenario is presented in evolutionary sources as an established fact. However, an in-depth study of the details and the scientific data indicates that the scenario is based more on imagination than reality.
How reptiles, as land-dwelling creatures, ever came to fly, is an issue which has stirred up considerable speculation among evolutionists. There are two main theories. The first argues that the ancestors of birds descended to the ground from the trees. As a result, these ancestors are alleged to be reptiles that lived in the treetops and came to possess wings gradually as they jumped from one branch to another. This is known as the arboreal theory. The other, the cursorial (or "running") theory, suggests that birds progressed to the air from the land.
Yet both of these theories rest upon speculative interpretations, and there is no evidence to support either of them. Evolutionists have devised a simple solution to the problem: they simply imagine that the evidence exists. Professor John Ostrom, head of the Geology Department at Yale University, who proposed the cursorial theory, explains this approach:
No fossil evidence exists of any pro-avis. It is a purely hypothetical pre-bird, but one that must have existed.106
However, this transitional form, which the arboreal theory assumes "must have lived," has never been found. The cursorial theory is even more problematic. The basic assumption of the theory is that the front legs of some reptiles gradually developed into wings as they waved their arms around in order to catch insects. However, no explanation is provided of how the wing, a highly complex organ, came into existence as a result of this flapping.
One huge problem for the theory of evolution is the irreducible complexity of wings. Only a perfect structure allows wings to function, a "half-way developed" wing cannot function. In this context, the "gradual development" model—the unique mechanism postulated by evolution—makes no sense. Thus Robert Carroll is forced to admit that, "It is difficult to account for the initial evolution of feathers as elements in the flight apparatus, since it is hard to see how they could function until they reached the large size seen in Archaeopteryx."107 Then he argues that feathers could have evolved for insulation, but this does not explain their complex structure which is specifically shaped for flying.
It is essential that wings should be tightly attached to the chest, and possess a structure able to lift the bird up and enable it to move in all directions, as well as allowing it to remain in the air. It is essential that wings and feathers possess a light, flexible and well proportioned structure. At this point, evolution is again in a quandary. It fails to answer the question of how this flawless anatomy of wings came about as the result of accumulative random mutations. Similarly, it offers no explanation of how the foreleg of a reptile came to change into a perfect wing as a result of a defect (mutation) in the genes.
A half-formed wing cannot fly. Consequently, even if we assume that mutation did lead to a slight change in the foreleg, it is still entirely unreasonable to assume that further mutations contributed coincidentally to the development of a full wing. That is because a mutation in the forelegs will not produce a new wing; on the contrary, it will just cause the animal to lose its forelegs. This would put it at a disadvantage compared to other members of its own species. According to the rules of the theory of evolution, natural selection would soon eliminate this flawed creature.
According to biophysical research, mutations are changes that occur very rarely. Consequently, it is impossible that a disabled animal could wait millions of years for its wings to fully develop by means of slight mutations, especially when these mutations have damaging effects over time…

Imaginary Theories, Imaginary Creatures
The first theory put forward by evolutionists to account for the origin of flight claimed that reptiles developed wings as they hunted flies (above); the second theory was that they turned into birds as they jumped from branch to branch (side). However, there are no fossils of animals which gradually developed wings, nor any discovery to show that such a thing could even be possible.
The theory of evolution holds that birds evolved from carnivorous and bipedal theropods. However, a comparison between birds and reptiles reveals that the two have very distinct features, making it unlikely that one evolved from the other.
There are various structural differences between birds and reptiles, one of which concerns bone structure. Due to their bulky natures, dinosaurs—the ancestors of birds according to evolutionists—had thick, solid bones. Birds, in contrast, whether living or extinct, have hollow bones that are very light, as they must be in order for flight to take place.
Another difference between reptiles and birds is their metabolic structure. Reptiles have the slowest metabolic structure in the animal kingdom. (The claim that dinosaurs had a warm-blooded fast metabolism remains a speculation.) Birds, on the other hand, are at the opposite end of the metabolic spectrum. For instance, the body temperature of a sparrow can rise to as much as 48°C due to its fast metabolism. On the other hand, reptiles lack the ability to regulate their body temperature. Instead, they expose their bodies to sunlight in order to warm up. Put simply, reptiles consume the least energy of all animals and birds the most.
One of the best-known ornithologists in the world, Alan Feduccia from the University of North Carolina, opposes the theory that birds are related to dinosaurs, despite the fact that he is an evolutionist himself. Feduccia has this to say regarding the reptile-bird scenario:
Well, I've studied bird skulls for 25 years and I don't see any similarities whatsoever. I just don't see it... The theropod origins of birds, in my opinion, will be the greatest embarrassment of paleontology of the 20th century.108
Larry Martin, a specialist on ancient birds from the University of Kansas, also opposes the theory that birds are descended from dinosaurs. Discussing the contradiction that evolution falls into on the subject, he states:
To tell you the truth, if I had to support the dinosaur origin of birds with those characters, I'd be embarrassed every time I had to get up and talk about it.109
Yet, despite all the scientific findings, the groundless scenario of "dinosaur-bird evolution" is still insistently advocated. Popular publications are particularly fond of the scenario. Meanwhile, concepts which provide no backing for the scenario are presented as evidence for the imaginary "dinosaur-bird evolution."
In some evolutionist publications, for instance, emphasis is laid on the differences among dinosaur hip bones to support the thesis that birds are descended from dinosaurs. These so-called differences exist between dinosaurs classified as Saurischian (reptile-like, hip-girdled species) and Ornithischian (bird-like, hip-girdled species). This concept of dinosaurs having hip girdles similar to those of birds is now and then taken as evidence for the alleged dinosaur–bird link. However, the difference in hip girdles is no evidence at all for the claim that birds evolved from dinosaurs. That is because Ornithischian dinosaurs do not resemble birds with respect to other anatomical features. For instance, Ankylosaurus is a dinosaur classified as Ornithischian, with short legs, a giant body, and skin covered with scales resembling armor. On the other hand, Struthiomimus, which resembles birds in some of its anatomical features (long legs, short forelegs, and thin structure), is actually a Saurischian.110
In short, the structure of the hip girdle is no evidence for an evolutionary relationship between birds and dinosaurs. The claim that dinosaurs resemble birds because their hip girdles are similar ignores other significant anatomical differences between the two species which make any evolutionary link between them untenable from the evolutionist viewpoint.
Birds' Unique Skeletal System
Unlike dinosaur and reptile bones, bird bones are hollow. This gives the body stability and lightness. Birds' skeletal structure is employed in designing airplanes, bridges and modern structures.
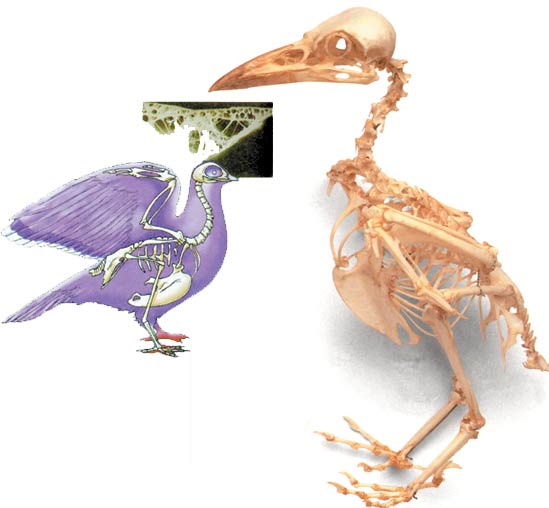
Dinosaur bones are thick and solid because of their massive structure, whereas the bones of living and extinct birds are hollow, and thus very light.
Another factor demonstrating the impossibility of the reptile-bird evolution scenario is the structure of avian lungs, which cannot be accounted for by evolution.
In land-dwelling creatures, air flow is bidirectional. Upon inhaling, the air travels through the passages in the lungs (bronchial tubes), ending in tiny air sacs (alveoli). The exchange of oxygen and carbon dioxide takes place here. Then, upon exhaling, this used air makes its way back and finds its way out of the lung by the same route.
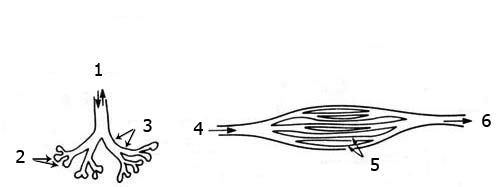
Reptile Lung
Avian Lung
1. Air Flow
2. Alveoli
3. Bronchi
4. Air Flows In
5. Parabronchi
6. Air Flows Out
Bird lungs function in a way that is completely contrary to the way the lungs of land animals function. The latter inhale and exhale through the same passages. The air in bird lungs, in contrast, passes continuously through the lung in one direction. This is made possible by special air sacs throughout the lung. By means of this system, whose details can be seen overleaf, birds breathe nonstop. This is peculiar to birds, which need high levels of oxygen during flight. It is impossible for this structure to have evolved from reptile lungs, because any creature with an "intermediate" form between the two types of lung would be unable to breathe.
In birds however, air is unidirectional. New air comes in one end, and the used air goes at the other end. By means of special air sacs all along the passages between them, air always flows in one direction through the avian lung. In this way, birds are able to take in air nonstop. This satisfies birds' high energy requirements. This highly specialized respiratory system is explained by Michael Denton in his book A Theory in Crisis:
In the case of birds, the major bronchi break down into tiny tubes which permeate the lung tissue. These so-called parabronchi eventually join up together again, forming a true circulatory system so that air flows in one direction through the lungs. ...[T]he structure of the lung in birds and the overall functioning of the respiratory system is quite unique. No lung in any other vertebrate species is known which in any way approaches the avian system. Moreover, it is identical in all essential details in birds as diverse as humming birds, ostriches and hawks.111
The important thing is that the reptile lung, with its bidirectional air flow, could not have evolved into the bird lung with its unidirectional flow, because it is not possible for there to have been an intermediate model between them. In order for a creature to live, it has to keep breathing, and a reversal of the structure of its lungs would inevitably end in death. According to evolution, this change must happen gradually over millions of years, whereas a creature whose lungs do not work will die within a few minutes.
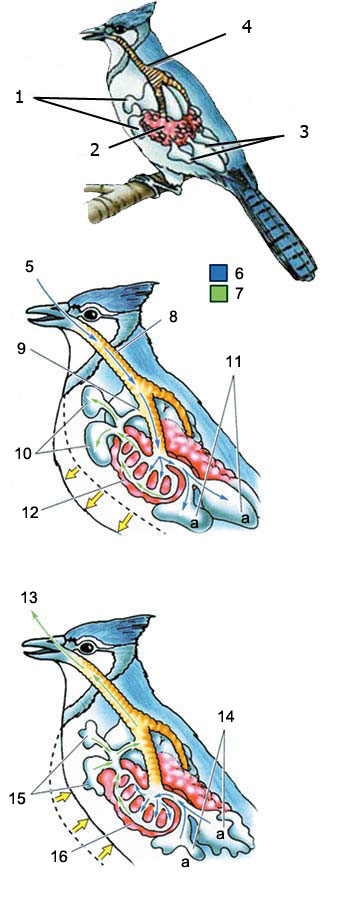
13. Stale air
14. Fresh air moves out of the rear air sacs to the lungs.
15. Stale air is expelled from the front air sacs.
16. Lung
Birds' Special Respiratory System
Breathing In: The air which enters birds' respiratory passages goes to the lungs, and to air sacs behind them. The air which is used is transferred to air sacs at the front.
Breathing Out: When a bird breathes out, the fresh air in the rear air sacs goes into the lungs. With this system, the bird is able to enjoy a constant supply of fresh air to its lungs.
There are many details in this lung system, which is shown in very simplified form in these diagrams. For instance, there are special valves where the sacs join the lungs, which enable the air to flow in the right direction. All of these show that there is clearly “creation” at work here. These special systems not only deal a death blow to the theory of evolution, they are also among the innumerable proofs of the fact of creation.
9. Fresh air does not pass through flont air sacs
10. Rear air sacs are filled with fresh air
11. Front air sacs are filled with the stale air coming from the lungs
12. Lung
Michael Denton states that it is impossible to give an evolutionary account of the avian lung:
Just how such an utterly different respiratory system could have evolved gradually from the standard vertebrate design is fantastically difficult to envisage, especially bearing in mind that the maintenance of respiratory function is absolutely vital to the life of an organism to the extent that the slightest malfunction leads to death within minutes. Just as the feather cannot function as an organ of flight until the hooks and barbules are coadapted to fit together perfectly, so the avian lung cannot function as an organ of respiration until the parabronchi system which permeates it and the air sac system which guarantees the parabronchi their air supply are both highly developed and able to function together in a perfectly integrated manner.112
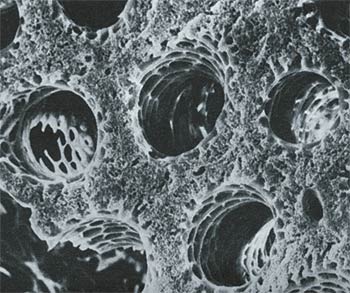
Parabronchial tubes, which enable air to circulate in the right direction in birds' lungs. Each of these tubes is just 0.5 mm. in diameter.
In brief, the passage from a terrestrial lung to an avian lung is impossible, because an intermediate form would serve no purpose.
Another point that needs to be mentioned here is that reptiles have a diaphragm-type respiratory system, whereas birds have an abdominal air sac system instead of a diaphragm. These different structures also make any evolution between the two lung types impossible, as John Ruben, an acknowledged authority in the field of respiratory physiology, observes in the following passage:
The earliest stages in the derivation of the avian abdominal air sac system from a diaphragm-ventilating ancestor would have necessitated selection for a diaphragmatic hernia in taxa transitional between theropods and birds. Such a debilitating condition would have immediately compromised the entire pulmonary ventilatory apparatus and seems unlikely to have been of any selective advantage.113
Another interesting structural feature of the avian lung which defies evolution is the fact that it is never empty of air, and thus never in danger of collapse. Michael Denton explains the position:
Just how such a different respiratory system could have evolved gradually from the standard vertebrate design without some sort of direction is, again, very difficult to envisage, especially bearing in mind that the maintenance of respiratory function is absolutely vital to the life of the organism. Moreover, the unique function and form of the avian lung necessitates a number of additional unique adaptations during avian development. As H. R. Dunker, one of the world's authorities in this field, explains, because first, the avian lung is fixed rigidly to the body wall and cannot therefore expand in volume and, second, because of the small diameter of the lung capillaries and the resulting high surface tension of any liquid within them, the avian lung cannot be inflated out of a collapsed state as happens in all other vertebrates after birth. The air capillaries are never collapsed as are the alveoli of other vertebrate species; rather, as they grow into the lung tissue, the parabronchi are from the beginning open tubes filled with either air or fluid.114
In other words, the passages in birds' lungs are so narrow that the air sacs inside their lungs cannot fill with air and empty again, as with land-dwelling creatures.
If a bird lung ever completely deflated, the bird would never be able to re-inflate it, or would at the very least have great difficulty in doing so. For this reason, the air sacs situated all over the lung enable a constant passage of air to pass through, thus protecting the lungs from deflating.
Of course this system, which is completely different from the lungs of reptiles and other vertebrates, and is based on the most sensitive equilibrium, cannot have come about with unconscious mutations, stage by stage, as evolution maintains. This is how Denton describes this structure of the avian lung, which again invalidates Darwinism:
The avian lung brings us very close to answering Darwin's challenge: "If it could be demonstrated that any complex organ existed, which could not possibly have been formed by numerous, successive, slight modifications, my theory would absolutely break down."115
Another impassable gulf between birds and reptiles is feathers, which are peculiar to birds. Reptile bodies are covered with scales, and those of birds with feathers. The hypothesis that bird feathers evolved from reptile scales is completely unfounded, and is indeed disproved by the fossil record, as the evolutionary paleontologist Barbara Stahl admits:
How [feathers] arose initially, presumably from reptiles scales, defies analysis... It seems, from the complex construction of feathers, that their evolution from reptilian scales would have required an immense period of time and involved a series of intermediate structures. So far, the fossil record does not bear out that supposition.116
A. H. Brush, a professor of physiology and neurobiology at the University of Connecticut, accepts this reality, although he is himself an evolutionist: "Every feature from gene structure and organization, to development, morphogenesis and tissue organization is different [in feathers and scales]."117 Moreover, Professor Brush examines the protein structure of bird feathers and argues that it is "unique among vertebrates."118
There is no fossil evidence to prove that bird feathers evolved from reptile scales. On the contrary, feathers appear suddenly in the fossil record, Professor Brush observes, as an "undeniably unique" character distinguishing birds.119 Besides, in reptiles, no epidermal tissue has yet been detected that provides a starting point for bird feathers.120
Many fossils have so far been the subject of "feathered dinosaur" speculation, but detailed study has always disproved it. The prominent ornithologist Alan Feduccia writes the following in an article called "On Why Dinosaurs Lacked Feathers":
Feathers are features unique to birds, and there are no known intermediate structures between reptilian scales and feathers. Notwithstanding speculations on the nature of the elongated scales found on such forms as Longisquama ... as being featherlike structures, there is simply no demonstrable evidence that they in fact are.121

The Sinosauropteryx fossil, announced by evolutionary paleontologists to be a "feathered dinosaur," but which subsequently turned out to be no such thing.
Reptile Scales
The scales that cover reptiles' bodies are totally different from bird feathers. Unlike feathers, scales do not extend under the skin, but are merely a hard layer on the surface of the animal's body. Genetically, biochemically and anatomically, scales bear no resemblance to feathers. This great difference between the two again shows that the scenario of evolution from reptiles to birds is unfounded.
On the other hand, bird feathers have such a complex structure that the phenomenon can never be accounted for by evolutionary processes. As we all know, there is a shaft that runs up the center of the feather. Attached to the shaft are the vanes. The vane is made up of small thread-like strands, called barbs. These barbs, of different lengths and rigidity, are what give the bird its aerodynamic nature. But what is even more interesting is that each barb has thousands of even smaller strands attached to them called barbules. The barbules are connected to barbicels, with tiny microscopic hooks, called hamuli. Each strand is hooked to an opposing strand, much like the hooks of a zipper.
Just one crane feather has about 650 barbs on each of side of the shaft. About 600 barbules branch off the barbs. Each one of these barbules are locked together with 390 hooklets. The hooks latch together as do the teeth on both sides of a zip. If the hooklets come apart for any reason, the bird can easily restore the feathers to their original form by either shaking itself or by straightening its feathers out with its beak.
The Complex Structure of Bird Feathers
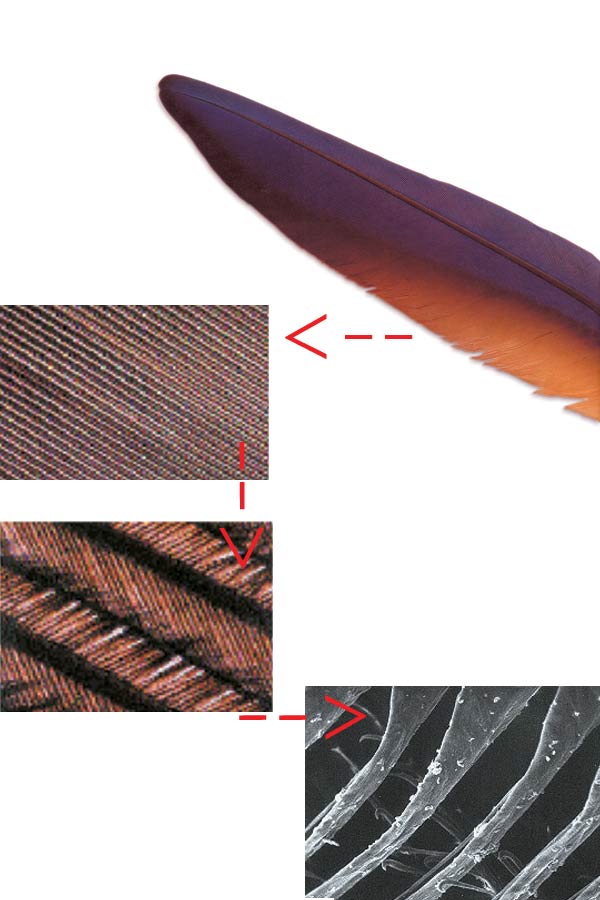
When bird feathers are studied closely, a very delicate design emerges. There are even tinier hairs on every tiny hair, and these have special hooks, allowing them to hold onto each other. The pictures show progressively enlarged bird feathers.
To claim that the complex structure of feathers could have come about by the evolution of reptile scales through chance mutations is quite simply a dogmatic belief with no scientific foundation. Even one of the doyens of Darwinism, Ernst Mayr, made this confession on the subject some years ago:
It is a considerable strain on one's credulity to assume that finely balanced systems such as certain sense organs (the eye of vertebrates, or the bird's feather) could be improved by random mutations.122
Feathers also compelled Darwin to ponder them. Moreover, the perfect aesthetics of the peacock's feathers had made him "sick" (his own words). In a letter he wrote to Asa Gray on April 3, 1860, he said, "I remember well the time when the thought of the eye made me cold all over, but I have got over this stage of complaint..." And then continued: "... and now trifling particulars of structure often make me very uncomfortable. The sight of a feather in a peacock's tail, whenever I gaze at it, makes me sick!"123
In short, the enormous structural differences between bird feathers and reptile scales, and the extraordinarily complex structure of feathers, clearly demonstrate the baselessness of the claim that feathers evolved from scales.

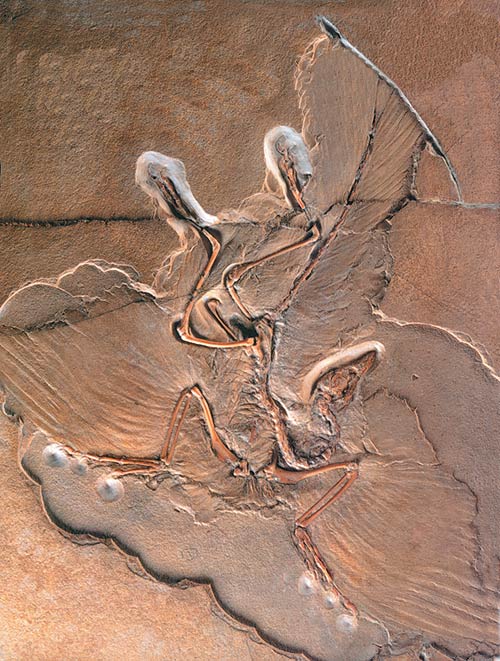
One of the important pieces of evidence that Archaeopteryx was a flying bird is its asymmetric feather structure. Above, one of the creature's fossil feathers.
In response to the question whether there is any fossil evidence for "reptile-bird evolution," evolutionists pronounce the name of one single creature. This is the fossil of a bird called Archaeopteryx, one of the most widely known so-called transitional forms among the very few that evolutionists still defend.
Archaeopteryx, the so-called ancestor of modern birds according to evolutionists, lived approximately 150 million years ago. The theory holds that some small dinosaurs, such as Velociraptors or Dromaeosaurs, evolved by acquiring wings and then starting to fly. Thus, Archaeopteryx is assumed to be a transitional form that branched off from its dinosaur ancestors and started to fly for the first time.
However, the latest studies of Archaeopteryx fossils indicate that this explanation lacks any scientific foundation. This is absolutely not a transitional form, but an extinct species of bird, having some insignificant differences from modern birds.
The thesis that Archaeopteryx was a "half-bird" that could not fly perfectly was popular among evolutionist circles until not long ago. The absence of a sternum (breastbone) in this creature was held up as the most important evidence that this bird could not fly properly. (The sternum is a bone found under the thorax to which the muscles required for flight are attached. In our day, this breastbone is observed in all flying and non-flying birds, and even in bats, a flying mammal which belongs to a very different family.) However, the seventh Archaeopteryx fossil, which was found in 1992, disproved this argument. The reason was that in this recently discovered fossil, the breastbone that was long assumed by evolutionists to be missing was discovered to have existed after all. This fossil was described in the journal Nature as follows:
The recently discovered seventh specimen of the Archaeopteryx preserves a partial, rectangular sternum, long suspected but never previously documented. This attests to its strong flight muscles, but its capacity for long flights is questionable.124
This discovery invalidated the mainstay of the claims that Archaeopteryx was a half-bird that could not fly properly.
Morevoer, the structure of the bird's feathers became one of the most important pieces of evidence confirming that Archaeopteryx was a flying bird in the true sense. The asymmetric feather structure of Archaeopteryx is indistinguishable from that of modern birds, and indicates that it could fly perfectly well. As the eminent paleontologist Carl O. Dunbar states, "Because of its feathers, [Archaeopteryx is] distinctly to be classed as a bird."125 Paleontologist Robert Carroll further explains the subject:
The geometry of the flight feathers of Archaeopteryx is identical with that of modern flying birds, whereas nonflying birds have symmetrical feathers. The way in which the feathers are arranged on the wing also falls within the range of modern birds… According to Van Tyne and Berger, the relative size and shape of the wing of Archaeopteryx are similar to that of birds that move through restricted openings in vegetation, such as gallinaceous birds, doves, woodcocks, woodpeckers, and most passerine birds… The flight feathers have been in stasis for at least 150 million years…126
National Geographic's great hit, the "dino-bird."
Archaeoraptor soon turned out to be a hoax.
Another fact that was revealed by the structure of Archaeopteryx's feathers was its warm-blooded metabolism. As was discussed above, reptiles and dinosaurs are cold-blooded animals whose body heat fluctuates with the temperature of their environment, rather than being homeostatically regulated. A very important function of the feathers on birds is the maintenance of a constant body temperature. The fact that Archaeopteryx had feathers shows that it was a real, warm-blooded bird that needed to retain its body heat, in contrast to dinosaurs.

Just like Archaeopteryx, there are claw-like nails on the wings of the bird Opisthocomus hoazin, which lives in our own time.
Two important points evolutionary biologists rely on when claiming Archaeopteryx was a transitional form, are the claws on its wings and its teeth.
It is true that Archaeopteryx had claws on its wings and teeth in its mouth, but these traits do not imply that the creature bore any kind of relationship to reptiles. Besides, two bird species living today, the touraco and the hoatzin, have claws which allow them to hold onto branches. These creatures are fully birds, with no reptilian characteristics. That is why it is completely groundless to assert that Archaeopteryx is a transitional form just because of the claws on its wings.
Neither do the teeth in Archaeopteryx's beak imply that it is a transitional form. Evolutionists are wrong to say that these teeth are reptilian characteristics, since teeth are not a typical feature of reptiles. Today, some reptiles have teeth while others do not. Moreover, Archaeopteryx is not the only bird species to possess teeth. It is true that there are no toothed birds in existence today, but when we look at the fossil record, we see that both during the time of Archaeopteryx and afterwards, and even until fairly recently, a distinct group of birds existed that could be categorised as "birds with teeth."
The most important point is that the tooth structure of Archaeopteryx and other birds with teeth is totally different from that of their alleged ancestors, the dinosaurs. The well-known ornithologists L. D. Martin, J. D. Stewart, and K. N. Whetstone observed that Archaeopteryx and other similar birds have unserrated teeth with constricted bases and expanded roots. Yet the teeth of theropod dinosaurs, the alleged ancestors of these birds, had serrated teeth with straight roots.127 These researchers also compared the ankle bones of Archaeopteryx with those of their alleged ancestors, the dinosaurs, and observed no similarity between them.128
Studies by anatomists such as S. Tarsitano, M.K. Hecht, and A.D. Walker have revealed that some of the similarities that John Ostrom and others have seen between the limbs of Archaeopteryx and dinosaurs were in reality misinterpretations.129 For example, A.D. Walker has analyzed the ear region of Archaeopteryx and found that it is identical to that of modern-day birds.130
Furthermore, J. Richard Hinchliffe, from the Institute of Biological Sciences of the University of Wales, studied the anatomies of birds and their alleged reptilian ancestors by using modern isotopic techniques and discovered that the three forelimb digits in dinosaurs are I-II-III, whereas bird wing digits are II-III-IV. This poses a big problem for the supporters of the Archaeopteryx-dinosaur link.131 Hinchliffe published his studies and observations in Science in 1997, where he wrote:
Doubts about homology between theropods and bird digits remind us of some of the other problems in the "dinosaur-origin" hypothesis. These include the following: (i) The much smaller theropod forelimb (relative to body size) in comparison with the Archaeopteryx wing. Such small limbs are not convincing as proto-wings for a ground-up origin of flight in the relatively heavy dinosaurs. (ii) The rarity in theropods of the semilunate wrist bone, known in only four species (including Deinonychus). Most theropods have relatively large numbers of wrist elements, difficult to homologize with those of Archaeopteryx. (iii) The temporal paradox that most theropod dinosaurs and in particular the birdlike dromaeosaurs are all very much later in the fossil record than Archaeopteryx.
As Hinchliffe notes, the "temporal paradox" is one of the facts that deal the fatal blow to the evolutionist allegations about Archaeopteryx. In his book Icons of Evolution, American biologist Jonathan Wells remarks that Archaeopteryx has been turned into an "icon" of the theory of evolution, whereas evidence clearly shows that this creature is not the primitive ancestor of birds. According to Wells, one of the indications of this is that theropod dinosaurs—the alleged ancestors of Archaeopteryx—are actually younger than Archaeopteryx: "Two-legged reptiles that ran along the ground, and had other features one might expect in an ancestor of Archaeopteryx, appear later."132
All these findings indicate that Archaeopteryx was not a transitional link but only a bird that fell into a category that can be called "toothed birds." Linking this creature to theropod dinosaurs is completely invalid. In an article headed "The Demise of the 'Birds Are Dinosaurs' Theory," the American biologist Richard L. Deem writes the following about Archaeopteryx and the bird-dinosaur evolution claim:
The results of the recent studies show that the hands of the theropod dinosaurs are derived from digits I, II, and III, whereas the wings of birds, although they look alike in terms of structure, are derived from digits II, III, and IV... There are other problems with the "birds are dinosaurs" theory. The theropod forelimb is much smaller (relative to body size) than that of Archaeopteryx. The small "proto-wing" of the theropod is not very convincing, especially considering the rather hefty weight of these dinosaurs. The vast majority of the theropod lack the semilunate wrist bone, and have a large number of other wrist elements which have no homology to the bones of Archaeopteryx. In addition, in almost all theropods, nerve V1 exits the braincase out the side, along with several other nerves, whereas in birds, it exits out the front of the braincase, though its own hole. There is also the minor problem that the vast majority of the theropods appeared after the appearance of Archaeopteryx.133

Confuciusornis, which lived at the same time as Archaeopteryx, has many similarities to modern birds.
Some recently found fossils also invalidate the evolutionist scenario regarding Archaeopteryx in other respects.
Lianhai Hou and Zhonghe Zhou, two paleontologists at the Chinese Institute of Vertebrate Paleontology, discovered a new bird fossil in 1995, and named it Confuciusornis. This fossil is almost the same age as Archaeopteryx (around 140 million years), but has no teeth in its mouth. In addition, its beak and feathers share the same features as today's birds. Confuciusornis has the same skeletal structure as modern birds, but also has claws on its wings, just like Archaeopteryx. Another structure peculiar to birds called the "pygostyle," which supports the tail feathers, was also found in Confuciusornis.134 In short, this fossil—which is the same age as Archaeopteryx, which was previously thought to be the earliest bird and was accepted as a semi-reptile—looks very much like a modern bird. This fact has invalidated all the evolutionist theses claiming Archaeopteryx to be the primitive ancestor of all birds.
Another fossil unearthed in China caused even greater confusion. In November 1996, the existence of a 130-million-year-old bird named Liaoningornis was announced in Science by L. Hou, L. D. Martin, and Alan Feduccia. Liaoningornis had a breastbone to which the muscles for flight were attached, just as in modern birds.135 This bird was indistinguishable from modern birds in other respects, too. The only difference was the teeth in its mouth. This showed that birds with teeth did not possess the primitive structure alleged by evolutionists. That Liaoningornis had the features of a modern bird was stated in an article in Discover, which said, "Whence came the birds? This fossil suggests that it was not from dinosaur stock."136
Another fossil that refuted the evolutionist claims regarding Archaeopteryx was Eoalulavis. The wing structure of Eoalulavis, which was said to be some 25 to 30 million years younger than Archaeopteryx, was also observed in modern slow-flying birds.137 This proved that 120 million years ago, there were birds indistinguishable from modern birds in many respects, flying in the skies.
These facts once more indicate for certain that neither Archaeopteryx nor other ancient birds similar to it were transitional forms. The fossils do not indicate that different bird species evolved from each other. On the contrary, the fossil record proves that today's modern birds and some archaic birds such as Archaeopteryx actually lived together at the same time. It is true that some of these bird species, such as Archaeopteryx and Confuciusornis, have become extinct, but the fact that only some of the species that once existed have been able to survive down to the present day does not in itself support the theory of evolution.
National Geographic dergisinin kuşların evrimi senaryosunun delili olarak tanıttığı Archaeoraptor adlı "dino-kuş"un, bir yıl sonra sahte bir fosil olduğu ortaya çıktı.
Unable to find what they were looking for in Archaeopteryx, the advocates of the theory of evolution pinned their hopes on some other fossils in the 1990s and a series of reports of so-called "dino-bird" fossils appeared in the world media. Yet it was soon discovered that these claims were simply misinterpretations, or, even worse, forgeries.
The first dino-bird claim was the story of "feathered dinosaur fossils unearthed in China," which was put forward in 1996 with a great media fanfare. A reptilian fossil called Sinosauropteryx was found, but some evolutionist paleontologists who examined the fossil said that it had bird feathers, unlike known reptiles. Examinations conducted one year later, however, showed that the fossil actually had no structure similar to a bird's feather. A Science article titled "Plucking the Feathered Dinosaur" stated that the structures named as "feathers" by evolutionary paleontologists definitely had nothing to do with feathers:
Exactly 1 year ago, paleontologists were abuzz about photos of a so-called "feathered dinosaur," which were passed around the halls at the annual meeting of the Society of Vertebrate Paleontology. The Sinosauropteryx specimen from the Yixian Formation in China made the front page of The New York Times, and was viewed by some as confirming the dinosaurian origins of birds. But at this year's vertebrate paleontology meeting in Chicago late last month, the verdict was a bit different: The structures are not modern feathers, say the roughly half-dozen Western paleontologists who have seen the specimens. ...Paleontologist Larry Martin of Kansas University, Lawrence, thinks the structures are frayed collagenous fibers beneath the skin—and so have nothing to do with birds.138
A yet more sensational case of dino-bird hype broke out in 1999. In its November 1999 issue, National Geographic published an article about a fossil specimen unearthed in China which was claimed to bear both bird and dinosaur features. National Geographic writer Christopher P. Sloan, the author of the article, went so far as to claim, "we can now say that birds are theropods just as confidently as we say that humans are mammals." This species, which was said to have lived 125 million years ago, was immediately given the scientific name Archaeoraptor liaoningensis.139
However, the fossil was a fake and was skillfully constructed from five separate specimens. A group of researchers, among whom were also three paleontologists, proved the forgery one year later with the help of X-ray computed tomography. The dino-bird was actually the product of a Chinese evolutionist. Chinese amateurs formed the dino-bird by using glue and cement from 88 bones and stones. Research suggests that Archaeoraptor was built from the front part of the skeleton of an ancient bird, and that its body and tail included bones from four different specimens.
The interesting thing is that National Geographic published a high-profile article about such a crude forgery—and, moreover, used it as the basis for claiming that "bird evolution" scenarios had been verified—without expressing any doubts or caution in the article at all. Dr. Storrs Olson, of the famous Smithsonian Institute Natural History Museum in the USA, later said that he warned National Geographic beforehand that this fossil was a fake, but that the magazine management totally ignored him. According to Olson, "National Geographic has reached an all-time low for engaging in sensationalistic, unsubstantiated, tabloid journalism."140
In a letter he wrote to Peter Raven of National Geographic, Olson describes the real story of the "feathered dinosaur" hype since its launch with a previous National Geographic article published in 1998 in a very detailed way:
Prior to the publication of the article "Dinosaurs Take Wing" in the July 1998 National Geographic, Lou Mazzatenta, the photographer for Sloan's article, invited me to the National Geographic Society to review his photographs of Chinese fossils and to comment on the slant being given to the story. At that time, I tried to interject the fact that strongly supported alternative viewpoints existed to what National Geographic intended to present, but it eventually became clear to me that National Geographic was not interested in anything other than the prevailing dogma that birds evolved from dinosaurs.
Sloan's article takes the prejudice to an entirely new level and consists in large part of unverifiable or undocumented information that "makes" the news rather than reporting it. His bald statement that "we can now say that birds are theropods just as confidently as we say that humans are mammals" is not even suggested as reflecting the views of a particular scientist or group of scientists, so that it figures as little more than editorial propagandizing. This melodramatic assertion had already been disproven by recent studies of embryology and comparative morphology, which, of course, are never mentioned.
More importantly, however, none of the structures illustrated in Sloan's article that are claimed to be feathers have actually been proven to be feathers. Saying that they are is little more than wishful thinking that has been presented as fact. The statement on page 103 that "hollow, hairlike structures characterize protofeathers" is nonsense considering that protofeathers exist only as a theoretical construct, so that the internal structure of one is even more hypothetical.
The hype about feathered dinosaurs in the exhibit currently on display at the National Geographic Society is even worse, and makes the spurious claim that there is strong evidence that a wide variety of carnivorous dinosaurs had feathers. A model of the undisputed dinosaur Deinonychus and illustrations of baby tyrannosaurs are shown clad in feathers, all of which is simply imaginary and has no place outside of science fiction.
This revealing case demonstrates two important facts. First, there are people who have no qualms about resorting to forgery in an effort to find evidence for the theory of evolution. Second, some highly reputable popular science journals, which have assumed the mission of imposing the theory of evolution on people, are perfectly willing to disregard any facts that may be inconvenient or have alternative interpretations. That is, they have become little more than propaganda tools for propagating the theory of evolution. They take not a scientific, but a dogmatic, stance and knowingly compromise science to defend the theory of evolution to which they are so strongly devoted.
Another important aspect of the matter is that there is no evidence for the thesis that birds evolved from dinosaurs. Because of the lack of evidence, either fake evidence is produced, or actual evidence is misinterpreted. In truth, there is no evidence that birds have evolved from another living species. On the contrary, all discoveries show that birds emerged on the earth already in full possession of their distinctive body structures.
While discussing the origin of birds, we mentioned the cursorial theory that evolutionary biologists propose. As we made clear then, the question of how reptiles grew wings involves speculation about "reptiles trying to catch insects with their front legs." According to this theory, these reptiles' forefeet slowly turned into wings over time as they hunted for insects.
Latest Evidence: Ostrich Study Refutes The Dino-Bird Story
The latest blow to the "birds evolved from dinosaurs" theory came from a study made on the embryology of ostriches.
Drs. Alan Feduccia and Julie Nowicki of the University of North Carolina at Chapel Hill studied a series of live ostrich eggs and, once again, concluded that there cannot be an evolutionary link between birds and dinosaurs. EurekAlert, a scientific portal held by the American Association for the Advancement of Science (AAAS), reports the following:
Drs. Alan Feduccia and Julie Nowicki of the University of North Carolina at Chapel Hill... opened a series of live ostrich eggs at various stages of development and found what they believe is proof that birds could not have descended from dinosaurs...
Whatever the ancestor of birds was, it must have had five fingers, not the three-fingered hand of theropod dinosaurs," Feduccia said... "Scientists agree that dinosaurs developed 'hands' with digits one, two and three... Our studies of ostrich embryos, however, showed conclusively that in birds, only digits two, three and four, which correspond to the human index, middle and ring fingers, develop, and we have pictures to prove it," said Feduccia, professor and former chair of biology at UNC. "This creates a new problem for those who insist that dinosaurs were ancestors of modern birds. How can a bird hand, for example, with digits two, three and four evolve from a dinosaur hand that has only digits one, two and three? That would be almost impossible." 1
In the same report, Dr. Freduccia also made important comments on the invalidity-and the shallowness-of the "birds evolved from dinosaurs" theory:
"There are insurmountable problems with that theory," he [Dr. Feduccia] said. "Beyond what we have just reported, there is the time problem in that superficially bird-like dinosaurs occurred some 25 million to 80 million years after the earliest known bird, which is 150 million years old."
If one views a chicken skeleton and a dinosaur skeleton through binoculars they appear similar, but close and detailed examination reveals many differences, Feduccia said. Theropod dinosaurs, for example, had curved, serrated teeth, but the earliest birds had straight, unserrated peg-like teeth. They also had a different method of tooth implantation and replacement."2
This evidence once again reveals that the "dino-bird" hype is just another "icon" of Darwinism: A myth that is supported only for the sake of a dogmatic faith in the theory.
1 - David Williamson, "Scientist Says Ostrich Study Confirms Bird 'Hands' Unlike Those Of Dinosaurs," EurekAlert, 14-Aug-2002, http://www.eurekalert.org/pub_releases/2002-08/uonc-sso081402.php
2 - David Williamson, "Scientist Says Ostrich Study Confirms Bird 'Hands' Unlike Those Of Dinosaurs," EurekAlert, 14-Aug-2002, http://www.eurekalert.org/pub_releases/2002-08/uonc-sso081402.php
Dr. Feduccia: His new study is enough to bury the 'dino-bird" myth
We have already stressed that this theory is based on no scientific discoveries whatsoever. But there is another interesting side to it, which we have not yet touched on. Flies can already fly. So how did they acquire wings? And generally speaking, what is the origin of insects, of which flies are just one class?

Winged insects emerge all of a sudden in the fossil record, and from that moment they have possessed the same flawless structures as today. The 320-million-year fossil dragonfly above is the oldest known specimen and is no different from dragonflies living today. No "evolution" has taken place.


Left: 145-million-year-old fossil fly. This fossil, found in Liaoning in China, is the same as flies of the same species living today.
Right: This Acantherpestes major millipede, found in the state of Kansas in the United States, is some 300 million years old, and no different from millipedes today.
In the classification of living things, insects make up a subphylum, Insecta, of the phylum Arthropoda. The oldest insect fossils belong to the Devonian Age (410 to 360 million years ago). In the Pennsylvanian Age which followed (325 to 286 million years ago), there emerged a great number of different insect species. For instance, cockroaches emerge all of a sudden, and with the same structure as they have today. Betty Faber, of the American Museum of Natural History, reports that fossil cockroaches from 350 million years ago are exactly the same as those of today.142
Creatures such as spiders, ticks, and millipedes are not insects, but rather belong to other subphyla of Arthropoda. Important fossil discoveries of these creatures were communicated to the 1983 annual meeting of the American Association for the Advancement of Science. The interesting thing about these 380-million-year-old spider, tick, and centipede fossils is the fact that they are no different from specimens alive today. One of the scientists who examined the fossils remarked that, "they looked like they might have died yesterday."143

There is no difference between this 320-million-year-old fossil cockroach and specimens living today.
Winged insects also emerge suddenly in the fossil record, and with all the features peculiar to them. For example, a large number of dragonfly fossils from the Pennsylvanian Age have been found. And these dragonflies have exactly the same structures as their counterparts today.
One interesting point here is the fact that dragonflies and flies emerge all of a sudden, together with wingless insects. This disproves the theory that wingless insects developed wings and gradually evolved into flying ones. In one of their articles in the book Biomechanics in Evolution, Robin Wootton and Charles P. Ellington have this to say on the subject:
When insect fossils first appear, in the Middle and Upper Carboniferous, they are diverse and for the most part fully winged. There are a few primitively wingless forms, but no convincing intermediates are known.144
One major characteristic of flies, which emerge all of a sudden in the fossil record, is their amazing flying technique. Whereas a human being is unable to open and close his arms even 10 times a second, a fly flaps its wings 500 times on average in that space of time. Moreover, it moves both its wings simultaneously. The slightest dissonance in the vibration of its wings would cause the fly to lose balance, but this never happens.
In an article titled "The Mechanical Design of Fly Wings," Wootton further observes:
The better we understand the functioning of insect wings, the more subtle and beautiful their designs appear … Structures are traditionally designed to deform as little as possible; mechanisms are designed to move component parts in predictable ways. Insect wings combine both in one, using components with a wide range of elastic properties, elegantly assembled to allow appropriate deformations in response to appropriate forces and to make the best possible use of the air. They have few if any technological parallels – yet.145
Of course the sudden emergence of living things with such a perfect structure as this cannot be explained by any evolutionist account. That is why Pierre-Paul Grassé says, "We are in the dark concerning the origin of insects."146 The origin of insects clearly proves the fact that all living things were created by Allah.
As we have stated before, the theory of evolution proposes that some imaginary creatures that came out of the sea turned into reptiles, and that birds evolved from reptiles. According to the same scenario, reptiles are the ancestors not only of birds, but also of mammals. However, there are great differences between these two classes. Mammals are warm-blooded animals (this means they can generate their own heat and maintain it at a steady level), they give live birth, they suckle their young, and their bodies are covered in fur or hair. Reptiles, on the other hand, are cold-blooded (i.e., they cannot generate heat, and their body temperature changes according to the external temperature), they lay eggs, they do not suckle their young, and their bodies are covered in scales.
Given all these differences, then, how did a reptile start to regulate its body temperature and come by a perspiratory mechanism to allow it to maintain its body temperature? Is it possible that it replaced its scales with fur or hair and started to secrete milk? In order for the theory of evolution to explain the origin of mammals, it must first provide scientific answers to these questions.
Yet, when we look at evolutionist sources, we either find completely imaginary and unscientific scenarios, or else a profound silence. One of these scenarios is as follows:
Some of the reptiles in the colder regions began to develop a method of keeping their bodies warm. Their heat output increased when it was cold and their heat loss was cut down when scales became smaller and more pointed, and evolved into fur. Sweating was also an adaptation to regulate the body temperature, a device to cool the body when necessary by evaporation of water. But incidentally the young of these reptiles began to lick the sweat of the mother for nourishment. Certain sweat glands began to secrete a richer and richer secretion, which eventually became milk. Thus the young of these early mammals had a better start in life.147
The above scenario is nothing more than a figment of the imagination. Not only is such a fantastic scenario unsupported by the evidence, it is clearly impossible. It is quite irrational to claim that a living creature produces a highly complex nutrient such as milk by licking its mother's body sweat.
The reason why such scenarios are put forward is the fact that there are huge differences between reptiles and mammals. One example of the structural barriers between reptiles and mammals is their jaw structure. Mammal jaws consist of only one mandibular bone containing the teeth. In reptiles, there are three little bones on both sides of the mandible. Another basic difference is that all mammals have three bones in their middle ear (hammer, anvil, and stirrup). Reptiles have but a single bone in the middle ear. Evolutionists claim that the reptile jaw and middle ear gradually evolved into the mammal jaw and ear. The question of how an ear with a single bone evolved into one with three bones, and how the sense of hearing kept on functioning in the meantime can never be explained. Not surprisingly, not one single fossil linking reptiles and mammals has been found. This is why Roger Lewin was forced to say, "The transition to the first mammal, ... is still an enigma."148
George Gaylord Simpson, one of the most important evolutionary authorities and a founder of the neo-Darwinist theory, makes the following comment regarding this perplexing difficulty for evolutionists:
The most puzzling event in the history of life on earth is the change from the Mesozoic, the Age of Reptiles, to the Age of Mammals. It is as if the curtain were rung down suddenly on the stage where all the leading roles were taken by reptiles, especially dinosaurs, in great numbers and bewildering variety, and rose again immediately to reveal the same setting but an entirely new cast, a cast in which the dinosaurs do not appear at all, other reptiles are supernumeraries, and all the leading parts are played by mammals of sorts barely hinted at in the preceding acts.149
Furthermore, when mammals suddenly made their appearance, they were already very different from each other. Such dissimilar animals as bats, horses, mice, and whales are all mammals, and they all emerged during the same geological period. Establishing an evolutionary relationship among them is impossible even by the broadest stretch of the imagination. The evolutionist zoologist R. Eric Lombard makes this point in an article that appeared in the leading journal Evolution:
Those searching for specific information useful in constructing phylogenies of mammalian taxa will be disappointed.150
In short, the origin of mammals, like that of other groups, fails to conform to the theory of evolution in any way. George Gaylord Simpson admitted that fact many years ago:
This is true of all thirty-two orders of mammals ... The earliest and most primitive known members of every order [of mammals] already have the basic ordinal characters, and in no case is an approximately continuous sequence from one order to another known. In most cases the break is so sharp and the gap so large that the origin of the order is speculative and much disputed ... This regular absence of transitional forms is not confined to mammals, but is an almost universal phenomenon, as has long been noted by paleontologists. It is true of almost all classes of animals, both vertebrate and invertebrate...it is true of the classes, and of the major animal phyla, and it is apparently also true of analogous categories of plants.151

There is no difference between fossil mammals dozens of millions of years old in natural history museums and those living today. Furthermore, these fossils emerge suddenly, with no connection to species that had gone before.
One important subject in the origin of mammals is the myth of the "evolution of the horse," also a topic to which evolutionist publications have devoted a considerable amount of space for a long time. This is a myth, because it is based on imagination rather than scientific findings.
Until recently, an imaginary sequence supposedly showing the evolution of the horse was advanced as the principal fossil evidence for the theory of evolution. Today, however, many evolutionists themselves frankly admit that the scenario of horse evolution is bankrupt. In 1980, a four-day symposium was held at the Field Museum of Natural History in Chicago, with 150 evolutionists in attendance, to discuss the problems with the gradualistic evolutionary theory. In addressing this meeting, evolutionist Boyce Rensberger noted that the scenario of the evolution of the horse has no foundation in the fossil record, and that no evolutionary process has been observed that would account for the gradual evolution of horses:
The popularly told example of horse evolution, suggesting a gradual sequence of changes from four-toed fox-sized creatures living nearly 50 million years ago to today's much larger one-toed horse, has long been known to be wrong. Instead of gradual change, fossils of each intermediate species appear fully distinct, persist unchanged, and then become extinct. Transitional forms are unknown.152
While discussing this important dilemma in the scenario of the evolution of the horse in a particularly honest way, Rensberger brought the transitional form difficulty onto the agenda as the greatest difficulty of all.

The Evolution of the Horse exhibition in London's Natural History Museum. This and other "evolution of the horse" diagrams show independent species which lived at different times and in different places, lined up one after the other in a very subjective presentation. In reality, there are no scientific discoveries regarding the evolution of the horse.
Dr. Niles Eldredge said the following about the "evolution of the horse" diagram:
There have been an awful lot of stories, some more imaginative than others, about what the nature of that history [of life] really is. The most famous example, still on exhibit downstairs, is the exhibit on horse evolution prepared perhaps fifty years ago. That has been presented as the literal truth in textbook after textbook. Now I think that is lamentable, particularly when the people who propose those kinds of stories may themselves be aware of the speculative nature of some of that stuff.153
Then what is the scenario of the evolution of the horse? This scenario was formulated by means of the deceitful charts devised by the sequential arrangement of fossils of distinct species that lived at vastly different periods in India, South Africa, North America, and Europe, solely in accordance with the rich power of evolutionists' imaginations. More than 20 charts of the evolution of the horse, which by the way are totally different from each other, have been proposed by various researchers. Thus, it is obvious that evolutionists have reached no common agreement on these family trees. The only common feature in these arrangements is the belief that a dog-sized creature called Eohippus (Hyracotherium), which lived in the Eocene period 55 million years ago, was the ancestor of the horse. However, the fact is that Eohippus, which became extinct millions of years ago, is nearly identical to the hyrax, a small rabbit-like animal which still lives in Africa and has nothing whatsoever to do with the horse.154
The inconsistency of the theory of the evolution of the horse becomes increasingly apparent as more fossil findings are gathered. Fossils of modern horse species (Equus nevadensis and Equus occidentalis) have been discovered in the same layer as Eohippus.155 This is an indication that the modern horse and its so-called ancestor lived at the same time.
The evolutionist science writer Gordon R. Taylor explains this little-acknowledged truth in his book The Great Evolution Mystery:
But perhaps the most serious weakness of Darwinism is the failure of paleontologists to find convincing phylogenies or sequences of organisms demonstrating major evolutionary change... The horse is often cited as the only fully worked-out example. But the fact is that the line from Eohippus to Equus is very erratic. It is alleged to show a continual increase in size, but the truth is that some variants were smaller than Eohippus, not larger. Specimens from different sources can be brought together in a convincing-looking sequence, but there is no evidence that they were actually ranged in this order in time.156
All these facts are strong evidence that the charts of horse evolution, which are presented as one of the most solid pieces of evidence for the theory of evolution, are nothing but fantastic and implausible fairy tales. Like other species, horses, too, came into existence without ancestors in the evolutionary sense.

Bats' sonar system is more sensitive and efficient than any technological sonar systems so far constructed.
One of the most interesting creatures in the mammalian class is without doubt the flying mammal, the bat.
Topping the list of the characteristics of bats is the complex "sonar" system they possess. By means of this, bats can fly in the pitch dark, unable to see anything, but performing the most complicated maneuvers. They can even sense and catch a caterpillar on the floor of a dark room.
Bat sonar works in the following way. The animal emits a continuous stream of high-frequency sonic signals, analyses the echoes from these, and as a result forms a detailed image of its surroundings. What is more, it manages to do all of this at an amazing speed, continually and unerringly, while it is flying through the air.
Research into the bat sonar system has produced even more surprising results. The range of frequencies the animal can perceive is very narrow; in other words it can only hear sounds of certain frequencies, which raises a very important point. Since sounds which strike a body in motion change their frequency (the well-known "Doppler effect"), as a bat sends out signals to a fly, say, that is moving away from it, the sound waves reflected from the fly should be at a different frequency that the bat is unable to perceive. For this reason, the bat should have great difficulty in sensing moving bodies.
But this is not the case. The bat continues to catch all kinds of small, fast-moving creatures with no difficulty at all. The reason is that the bat adjusts the frequency of the sound waves it sends out toward the moving bodies in its environment as if it knew all about the Doppler effect. For instance, it emits its highest-frequency signal toward a fly that is moving away from it, so that when the signal comes back, its frequency has not dropped below the threshold of the animal's hearing.
So how does this adjustment take place?
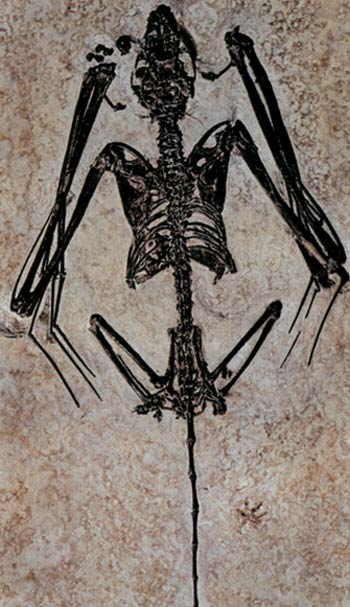
The oldest known fossil bat, found in Wyoming in the United States. 50 million years old, there is no difference between this fossil and bats alive today.
There are two groups of neurons (nerve cells) in the bat's brain which control the sonar system. One of these perceives the echoed ultrasound, and the other gives instructions to the muscles to produce echolocation calls. These regions in the brain work in tandem, in such a way that when the frequency of the echo changes, the first region perceives this, and warns the second one, enabling it to modify the frequency of the sound emitted in accordance with that of the echo. As a result, the pitch of the bat's ultrasound changes according to its surroundings, and sonar system as a whole is used in the most efficient manner.
It is impossible to be blind to the mortal blow that the bat sonar system deals to the theory of gradual evolution through chance mutations. It is an extremely complex structure, and can in no way be accounted for by chance mutations. In order for the system to function at all, all of its components have to work together perfectly as an integrated whole. It is absurd to believe that such a highly integrated system can be explained by chance; on the contrary, it actually demonstrates that the bat is flawlessly created.
In fact, the fossil record also confirms that bats emerged suddenly and with today's complex structures. In their book Bats: A Natural History, the evolutionary paleontologists John E. Hill and James D. Smith reveal this fact in the form of the following admission:
The fossil record of bats extends back to the early Eocene ... and has been documented ... on five continents ... [A]ll fossil bats, even the oldest, are clearly fully developed bats and so they shed little light on the transition from their terrestrial ancestor.157
And the evolutionary paleontologist L. R. Godfrey has this to say on the same subject:
There are some remarkably well preserved early Tertiary fossil bats, such as Icaronycteris index, but Icaronycteris tells us nothing about the evolution of flight in bats because it was a perfectly good flying bat.158
Evolutionist scientist Jeff Hecht confesses the same problem in a 1998 New Scientist article:
[T]he origins of bats have been a puzzle. Even the earliest bat fossils, from about 50 million years ago, have wings that closely resemble those of modern bats.159
In short, bats' complex bodily systems cannot have emerged through evolution, and the fossil record demonstrates that no such thing happened. On the contrary, the first bats to have emerged in the world are exactly the same as those of today. Bats have always existed as bats.
Whales and dolphins belong to the order of marine mammals known as Cetacea. These creatures are classified as mammals because, just like land-dwelling mammals, they give live birth to their young and nurse them, they have lungs to breathe with, and they regulate their body temperature. For evolutionists, the origin of marine mammals has been one of the most difficult issues to explain. In many evolutionist sources, it is asserted that the ancestors of cetaceans left the land and evolved into marine mammals over a long period of time. Accordingly, marine mammals followed a path contrary to the transition from water to land, and underwent a second evolutionary process, returning to the water. This theory both lacks paleontological evidence and is self-contradictory. Thus, evolutionists have been silenced on this issue for a long time.
However, an evolutionist hype about the origin of marine mammals broke out in the 90's, argued to be based on some new fossil findings of the 80's like Pakicetus and Ambulocetus. These evidently quadrupedal and terrestrial extinct mammals were alleged to be the ancestors of whales and thus many evolutionist sources did not hesitate to call them "walking whales." (In fact the full name, Ambulocetus natans, means "walking and swimming whale.") Popular means of evolutionist indoctrination further vulgarized the story. National Geographic in its November 2001 issue, finally declared the full evolutionist scenario on the "Evolution of Whales."
Nevertheless, the scenario was based on evolutionist prejudice, not scientific evidence.
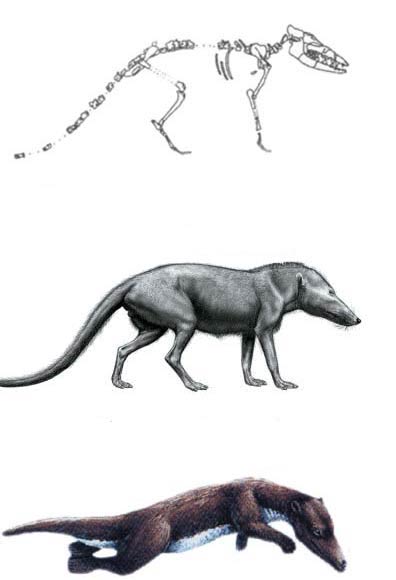
Fossil remains of the extinct mammal Pakicetus inachus, to give it its proper name, first came onto the agenda in 1983. P. D. Gingerich and his assistants, who found the fossil, had no hesitation in immediately claiming that it was a "primitive whale," even though they actually only found a skull.
Yet the fossil has absolutely no connection with the whale. Its skeleton turned out to be a four-footed structure, similar to that of common wolves. It was found in a region full of iron ore, and containing fossils of such terrestrial creatures as snails, tortoises, and crocodiles. In other words, it was part of a land stratum, not an aquatic one.
Pakicetus
Pakicetus reconstruction by National Geographic
Distortions in the Reconstructions of National Geographic
Paleontologists believe that Pakicetus was a quadrupedal mammal. The skeletal structure on the left, published in the Nature magazine (vol. 412, September 20, 2001) clearly demonstrates this. Thus the reconstruction of Pakicetus (below left) by Carl Buell, which was based on that structure, is realistic.
National Geographic, however, opted to use a picture of a "swimming" Pakicetus (below) in order to portray the animal as a "walking whale" and to impose that image on its readers. The inconsistencies in the picture, intended to make Pakicetus seem "whale-like," are immediately obvious: The animal has been portrayed in a "swimming" position. Its hind legs are shown stretching out backwards, and an impression of "fins" has been given.
So, how was a quadrupedal land dweller announced to be a "primitive whale"?Merely based on some details in its teeth and ear bones! These features, however, are not evidence on which to base a link between Pakicetus and the whale.
Even evolutionists admit that most of the theoretical relationships built on the basis of anatomical similarities between animals are completely untrustworthy. If the platypus, a billed mammal, and the duck had both been extinct for a long time, then there is no doubt that evolutionists would define them as very close relatives, based on the similiarity between their bills. However, since platypus is a mammal and duck is a bird, the theory of evolution cannot establish any link between the two, either.
Pakicetus, which evolutionists declare to be a "walking whale," was a unique species harboring different features in its body. In fact, Carroll, an authority on vertebrate paleontology, describes the Mesonychid family, of which Pakicetus should be a member, as "exhibiting an odd combination of characters."160 Even leading evolutionists such as Gould admit that such "mosaic creatures" cannot be regarded as evolutionary intermediate forms.
In his article "The Overselling of Whale Evolution," the creationist writer Ashby L. Camp reveals the total invalidity of the claim that the Mesonychid class, which should include land mammals such as Pakicetus, could have been the ancestors of Archaeocetea, or extinct whales, in these words:
The reason evolutionists are confident that mesonychids gave rise to archaeocetes, despite the inability to identify any species in the actual lineage, is that known mesonychids and archaeocetes have some similarities. These similarities, however, are not sufficient to make the case for ancestry, especially in light of the vast differences. The subjective nature of such comparisons is evident from the fact so many groups of mammals and even reptiles have been suggested as ancestral to whales.161
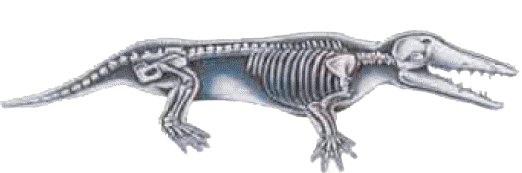
The second fossil creature after Pakicetus in the scenario on whale origins is Ambulocetus natans. It is actually a land creature that evolutionists have insisted on turning into a whale.
The name Ambulocetus natans comes from the Latin words "ambulare" (to walk), "cetus" (whale) and "natans" (swimming), and means "a walking and swimming whale." It is obvious the animal used to walk because it had four legs, like all other land mammals, and even wide claws on its feet and paws on its hind legs. Apart from evolutionists' prejudice, however, there is absolutely no basis for the claim that it swam in water, or that it lived on land and in water (like an amphibian).

National Geographic's Ambulocetus: The animal's rear legs are shown not with feet that would help it to walk, but as fins that would assist it to swim. However, Carroll, who examines the animal's leg bones, says that it possessed the ability to move powerfully on land.
In order to see the border between science and wishful imagination on this subject, let us have a look at National Geographic's reconstruction of Ambulocetus. This is how it is portrayed in the magazine:
If you look at it carefully you can easily see the two little visual manipulations that have been employed to turn the land-dwelling Ambulocetus into a whale:
• The animal's rear legs are shown not with feet that would help it to walk, but as fins that would assist it to swim. However, Carroll, who examined the animal's leg bones, says that it possessed the ability to move powerfully on land.162
• In order to present a flipper-like impression, webbing has been drawn on its front feet. Yet it is impossible to draw any such conclusion from a study of Ambulocetus fossils. In the fossil record it is next to impossible to find soft tissues such as these. So reconstructions based on features beyond those of the skeleton are always speculative. That offers evolutionists a wide-ranging empty space of speculation to use their propaganda tools.
With the same kind of evolutionists touching up that has been applied to the above Ambulocetus drawing, it is possible to make any animal look like any other. You could even take a monkey skeleton, draw fins on its back and webbing between its fingers and present it as the "primate ancestor of whales."
The invalidity of the deception carried out on the basis of the Ambulocetus fossil can be seen from the drawing below, published in the same issue of National Geographic:
In publishing the picture of the animal's skeleton, National Geographic had to take a step back from the retouching it had carried out to the reconstruction picture which made it seem more like a whale. As the skeleton clearly shows, the animal's foot bones were structured to carry it on land. There was no sign of the imaginary webs.
The real Ambulocetus : The legs are real legs, not "fins," and there are no imaginary webs between its toes such as National Geographic had added. (Picture from Carroll, Patterns and Processes of Vertebrate Evolution, p. 335)
In fact, there is no evidence that Pakicetus and Ambulocetus are ancestors of whales. They are merely described as "possible ancestors," based on some limited similarities, by evolutionists keen to find a terrestrial ancestor for marine mammals in the light of their theory. There is no evidence linking these creatures with the marine mammals that emerge in the fossil record at a very similar geological time.
After Pakicetus and Ambulocetus, the evolutionist plan moves on to the sea mammals and sets out (extinct whale) species such as Procetus, Rodhocetus, and Archaeocetea. The animals in question were mammals that lived in the sea and which are now extinct. (We shall be touching on this matter later.) However, there are considerable anatomical differences between these and Pakicetus and Ambulocetus. When we look at the fossils, it is clear they are not "transitional forms" linking each other:
• The backbone of the quadrupedal mammal Ambulocetus ends at the pelvis, and powerful rear legs then extend from it. This is typical land-mammal anatomy. In whales, however, the backbone goes right down to the tail, and there is no pelvic bone at all. In fact, Basilosaurus, believed to have lived some 10 million years after Ambulocetus, possesses the latter anatomy. In other words, it is a typical whale. There is no transitional form between Ambulocetus, a typical land mammal, and Basilosaurus, a typical whale.
• Under the backbone of Basilosaurus and the sperm whale, there are small bones independent of it. Evolutionists claim these to be vestigial legs. Yet in Basilosaurus, these bones functioned as copulary guides and in sperm whales "[act] as an anchor for the muscles of the genitalia."163 To describe these bones, which actually carry out important functions, as "vestigial organs" is nothing but Darwinistic prejudice.
In conclusion, the fact that there were no transitional forms between land and sea mammals and that they both emerged with their own particular features has not changed. There is no evolutionary link. Robert Carroll accepts this, albeit unwillingly and in evolutionist language: "It is not possible to identify a sequence of mesonychids leading directly to whales."164
Although he is an evolutionist, the famous Russian whale expert G. A. Mchedlidze, too, does not support the description of Pakicetus, Ambulocetus natans, and similar four-legged creatures as "possible ancestors of the whale," and describes them instead as a completely isolated group.165
Any evolutionary scenario between land and sea mammals has to explain the different ear and nose structures between the two groups. Let us first consider the ear structure. Like us, land mammals trap sounds from the outside world in the outer ear, amplify them with the bones in the middle ear, and turn them into signals in the inner ear. Marine mammals have no ear. They hear sounds by means of vibration-sensitive receptors in their lower jaws. The crucial point is that any evolution by stages between one perfect aural system to a completely different one is impossible. The transitional phases would not be advantageous. An animal that slowly loses its ability to hear with its ears, but has still not developed the ability to hear through its jaw, is at a disadvantage.
The question of how such a "development" could come about is an insoluble dilemma for evolutionists. The mechanisms evolutionists put forward are mutations and these have never been seen to add unequivocally new and meaningful information to animals' genetic information. It is unreasonable to suggest that the complex hearing system in sea mammals could have emerged as the result of mutations.
In fact, fossils show that no evolution ever happened. The ear system of Pakicetus and Ambulocetus is the same as that in terrestrial mammals. Basilosaurus, which follows these two land mammals in the supposed "evolutionary tree," on the other hand, possesses a typical whale ear. It was a creature that perceived sounds around it not through an outer ear but by vibrations reaching its jaw. And there is no "transitional form" between Basilosaurus' ear and that of Pakicetus and Ambulocetus.
A similar situation applies to the "sliding nose" tale. Evolutionist sources set out three skulls from Pakicetus, Rodhocetus and a grey whale from our own time above one another and claim that these represent an "evolutionary process." Whereas the three fossils' nasal structures, especially those of Rodhocetus and the grey whale are so different that it is impossible to accept them as transitional forms in the same series.
Furthermore, the movement of the nostrils to the forehead would require a "change" in the anatomy of the animals in question, and believing that this could happen as the result of random mutations is nothing but fantasy.
Many evolutionists maintain a kind of superstition about the origin of living things. This superstition is the magical "natural force" that allows living things to acquire the organs, biochemical structures, or anatomical features that they need. Let us have a look at a few interesting passages from National Geographic's article "Evolution of Whales":
… I tried to visualize some of the varieties of whale ancestors that had been found here and nearby... As the rear limbs dwindled, so did the hip bones that supported them… The neck shortened, turning the leading end of the body into more of a tubular hull to plow through the water with minimum drag, while arms assumed the shape of rudders. Having little need for outer ears any longer, some whales were receiving waterborne sounds directly through their lower jawbones and transmitting them to the inner ears via special fat pads.166
On close inspection, in this whole account the evolutionist mentality says that living things feel changing needs according to the changing environment they live in, and this need is perceived as an "evolutionary mechanism." According to this logic, less needed organs disappear, and needed organs appear of their own accord!
Anyone with the slightest knowledge of biology will know that our needs do not shape our organs hereditarily. Ever since Lamarck's theory of the transfer of acquired characteristics to subsequent generations was disproved, in other words for a century or so, that has been a known fact. Yet when one looks at evolutionist publications, they still seem to be thinking along Lamarckian lines. If you object, they will say: "No, we do not believe in Lamarck. What we say is that natural conditions put evolutionary pressure on living things, and that as a result of this, appropriate traits are selected, and in this way species evolve." Yet here lies the critical point: What evolutionists call "evolutionary pressure" cannot lead to living things acquiring new characteristics according to their needs. That is because the two so-called evolutionary mechanisms that supposedly respond to this pressure, natural selection and mutation, cannot provide new organs for animals:
• Natural selection can only select characteristics that already exist, it cannot create new ones.
• Mutations cannot add to the genetic information, they can only destroy the existing one. No mutation that adds unequivocally new, meaningful information to the genome (and which thus forms a new organ or new biochemical structure) has ever been observed.
If we look at the myth of National Geographic's awkwardly moving whales one more time in the light of this fact, we see that they are actually engaging in a rather primitive Lamarckism. On close inspection, National Geographic writer Douglas H. Chadwick "visualizes" that "the rear limbs dwindled" in each whale in the sequence. How could a morphological change happen in a species over generations in one particular direction? In order for that to happen, representatives of that species in every "sequence" would have to undergo mutations to shorten their legs, that mutation would have to cause the animals no other harm, those thus mutants would have to enjoy an advantage over normal ones, the next generations, by a great coincidence, would have to undergo the same mutation at the same point in its genes, this would have to carry on unchanged for many generations, and all of the above would have to happen by chance and quite flawlessly.
If the National Geographic writers believe that, then they will also believe someone who says: "My family enjoys flying. My son underwent a mutation and a few structures like bird feathers developed under his arms. My grandson will undergo the same mutation and the feathers will increase. This will go on for generations, and eventually my descendants will have wings and be able to fly." Both stories are equally ridiculous.
As we mentioned at the beginning, evolutionists display the superstition that living things' needs can be met by a magical force in nature. Ascribing consciousness to nature, a belief encountered in animist cultures, is interestingly rising up before our eyes in the 21st century under a "scientific" cloak. However, as the well-known French biologist Paul Pierre Grassé, a foremost critic of Darwinism, has once made it clear, "There is no law against daydreaming, but science must not indulge in it."167
Another scenario which evolutionists are trying to impose, without too much discussion, concerns the body surface of the animals in question. Like other mammals, Pakicetus and Ambulocetus, which are accepted as land mammals, are generally agreed to have had fur-covered bodies. And they are both shown as covered in thick fur in reconstructions. Yet when we move on to later animals (true marine mammals), all the fur disappears. The evolutionist explanation of this is no different from the fantastical Lamarckian-type scenarios we have seen above.
The truth of the matter is that all the animals in question were created in the most appropriate manner for their environments. It is irrational to try to account for them by means of mutation or facile Lamarckian stories. Like all features of life, the perfect systems in these creatures manifest the fact that they were created by Allah.

We have so far examined the fallacy of the evolutionist scenario that marine mammals evolved from terrestrial ones. Scientific evidence shows no relationship between the two terrestrial mammals (Pakicetus and Ambulocetus), that evolutionists put at the beginning of the story, and the marine mammals. So what about the rest of the scenario?
The theory of evolution is again in a great difficulty here. The theory tries to establish a phylogenetic link between Archaeocetea (archaic whales), sea mammals known to be extinct, and living whales and dolphins. However, evolutionary paleontologist Barbara J. Stahl admits that; "the serpentine form of the body and the peculiar serrated cheek teeth make it plain that these archaeocetes could not possibly have been ancestral to any of the modern whales."168
The evolutionist account of the origin of marine mammals faces a huge impasse in the form of discoveries in the field of molecular biology. The classical evolutionist scenario assumes that the two major whale groups, the toothed whales (Odontoceti) and the baleen whales (Mysticeti), evolved from a common ancestor. Yet Michel Milinkovitch of the University of Brussels has opposed this view with a new theory. He stresses that this assumption, based on anatomical similarities, is disproved by molecular discoveries:
Evolutionary relationships among the major groups of cetaceans is more problematic since morphological and molecular analyses reach very different conclusions. Indeed, based on the conventional interpretation of the morphological and behavioral data set, the echolocating toothed whales (about 67 species) and the filter-feeding baleen whales (10 species) are considered as two distinct monophyletic groups... On the other hand, phylogenetic analysis of DNA... and amino acid... sequences contradict this long-accepted taxonomic division. One group of toothed whales, the sperm whales, appear to be more closely related to the morphologically highly divergent baleen whales than to other odontocetes.169
In short, marine mammals defy the imaginary evolutionary scenarios which they are being forced to fit.
Contrary to the claims of evolutionist propaganda on the origin of marine mammals, we are dealing not with an evolutionary process backed up by empirical evidence, but by evidence coerced to fit a presupposed evolutionary family tree, despite the many contradictions between the two.
What emerges, if the evidence is looked at objectively, is that different living groups emerged independently of each other in the past. This is compelling empirical evidence of the fact that all of these creatures were created.
Mammals are regarded as the life forms on the top rungs of the so-called evolutionary ladder. That being the case, it is hard to explain why these animals moved over to a marine environment. Another question is how these creatures adapted to the marine environment even better than fish, since animals such as the killer whale and the dolphin, which are mammals and therefore possess lungs, are even better adapted to the environment they live in than fish that breathe in water.
It is perfectly obvious that the imaginary evolution of marine mammals cannot be explained in terms of mutations and natural selection. One article published in GEO magazine refers to the origin of the blue whale, a marine mammal, and states the despairing position of Darwinism on the subject thus:
Like blue whales, the bodily structures and organs of other mammals living in the sea also resemble those of fish. Their skeletons also bear similarities to those of fish. In whales, the rear limbs that we can refer to as legs exhibited a reverse development and did not reach full growth. Yet there is not the slightest information about these animals' form changes. We have to assume that the return to the sea took place not through a long-term, slow transition as claimed by Darwinism, but in momentary leaps. Paleontologists today lack sufficient information as regards which mammal species whales are evolved from.170
It is indeed very difficult to imagine how a small mammal living on dry land turned into a whale 30 meters in length and weighing some 60 tons. All that Darwinists can do in this regard is to produce figments of the imagination, as with the following extract from an article published in National Geographic:
The Whale's ascendancy to sovereign size apparently began sixty million years ago when hairy, four-legged mammals, in search of food or sanctuary, ventured into water. As eons passed, changes slowly occurred. Hind legs disappeared, front legs changed into flippers, hair gave way to a thick smooth blanket of blubber, nostrils moved to the top of the head, the tail broadened into flukes, and in the buoyant water world the body became enormous.171
The scenarios of gradual evolution described above satisfy nobody, not even their own authors. But let us in any case examine the details of this tale stage by stage in order to see just how unrealistic it actually is.

Marine mammals possess systems which are entirely peculiar to themselves. These are created in the most suitable way for the environment they live in.
To see the impossibility of the evolutionist scenario on the marine mammals, let us briefly examine some other unique features of these animals. When the adaptations a land-dwelling mammal has to undergo in order to evolve into a marine mammal are considered, even the word "impossible" seems inadequate. During such a transition, if even of one of the intermediary stages failed to happen, the creature would be unable to survive, which would put an end to the entire process. The adaptations that marine mammals must undergo during the transition to water are as follows:
1- Water-retention: Unlike other marine animals, marine mammals cannot use sea water to meet their water needs. They need fresh water to survive. Though we have limited information about the freshwater resources of marine mammals, it is believed that they feed on organisms containing a relatively low proportion of salt (about one third that of sea water). Thus, for marine mammals the retention of water in their bodies is crucial. That is why they have a water retention mechanism similar to that of camels. Like camels, marine mammals do not sweat; however, their kidneys are perfectly functional, producing highly concentrated urine that enables the animal to save water. In this way, water loss is reduced to a minimum.
Water retention can be seen even in minor details. For instance, the mother whale feeds her baby with a concentrated form of milk similar to cheese. This milk contains ten times more fat than human milk. There are a number of chemical reasons why this milk is so rich in fat. Water is released as the young whale digests the milk. In this way, the mother meets the young whale's water needs with minimum water loss.
2- Sight and communication: The eyes of dolphins and whales enable them to have acute eyesight in different environments. They have perfect eyesight in water as well as out. Yet most living things, including man, have poor eyesight out of their natural environments.
The eyes of marine and land-dwelling mammals are astonishingly elaborate. On land, the eyes face a number of potential dangers. That is why the eyes of land-dwelling animals have lids to protect them. In the ocean, the greatest threats to the eye come from the high level of salt and the pressure from currents. To avoid direct contact with the currents, the eyes are located on the sides of the head. In addition to this, a hard layer protects the eyes of creatures which dive to great depths. The eyes of marine mammals are equipped with elaborate features enabling them to see at depths where there is little light. For example, their lenses are perfectly circular in shape, while in their retinas, rods (the cells sensitive to light) outnumber cones (the cells sensitive to colours and details). Furthermore, the eyes of cetaceans also contain a phosphorus layer, which also helps them see particularly well in the dark.
The Great Morphological Differences Between Animals Which Are Claimed To Have Descended From One Another
So far, we have seen that different species emerged on earth with no evolutionary "intermediate forms" between them. They appear in the fossil record with such great differences that it is impossible to establish any evolutionary connection between them.
When we compare their skeletal structures, this fact can once again clearly be seen. Animals which are alleged to be evolutionary relatives differ enormously. We shall now examine some examples of these. All the drawings have been taken from evolutionist sources by experts on vertebrates. (As also contrasted by Michael Denton in his Evolution:A Theory in Crisis, 1986)

The marine reptile Mesosaurus, alleged to have evolved from Hylonomus.
The marine reptile Ichthyosaurus, alleged to have evolved from Hylonomus.
Hylonomus, the oldest known marine reptile.
Two different species of marine reptiles, and the land animal that evolutionists claim is their nearest ancestor. Take note of the great differences between them.
Even so, however, sight is not most important sensory modality of marine mammals. They rely more on their sense of hearing than is typically the case with land-dwelling mammals. Light is essential for sight, whereas hearing requires no such assistance. Many whales and dolphins hunt at a depth where it is completely dark, by means of a sonar mechanism they possess. Toothed whales, in particular, "see" by means of sound waves. Just as happens with light waves in the visual system, sound waves are focused and then analyzed and interpreted in the brain. This gives the cetacean accurate information regarding the shape, size, speed and position of the object in front of it. This sonic system is extremely sensitive—for instance, a dolphin can sense a person jumping into the sea. Sound waves are also used for determining direction and for communication. For example, two whales hundreds of kilometers apart can communicate via sound.

The oldest known Plesiosaurus skeleton
Skeleton of Araeoscelis, a Lower Permian reptile.
Plesiosaurus, the oldest known marine reptile, and its nearest terrestrial relative according to evolutionists. There is no resemblance between the two. The terrestrial reptile Araeocelis, regarded as the nearest ancestor of Plesiosaur by evolutionist authorities.
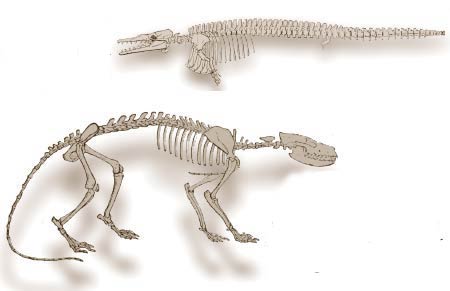
A typical example of the oldest known whales, Zygorhiza kochii, from the Eocene.
The ancestors of the whale are a subject of debate among evolutionist authorities, but some of them have decided on Ambulocetus. To the side is Ambulocetus, a typical tetrapod.
An early whale and what evolutionists claim to be its closest ancestor. Note that there is no resemblance between them. Even the best candidate that evolutionists have found for being the ancestor of whales has nothing to do with them.
The question of how these animals produce the sounds that enable them to determine direction or to communicate is still largely unresolved. As far as we know, one particular feature in the dolphin's body deserves particular attention: namely, the animal's skull is insulated against sound, a feature that protects the brain from continuous and intensive noise bombardment.
Let us now consider the question: Is it possible that all these astonishing features in marine mammals came into existence by means of natural selection and mutation? What mutation could result in the dolphin's body's coming to possess a sonar system and a brain insulated from sound? What kind of mutation could enable its eye to see in dark water? What mutation could lead to the mechanism that allows the most economic use of water?
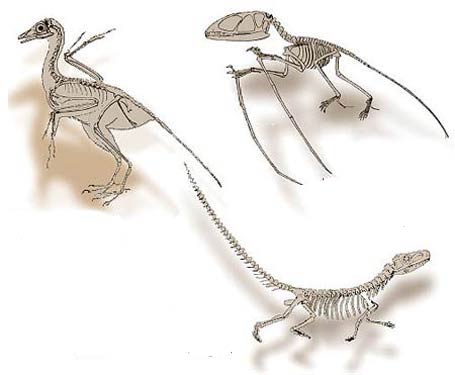
Dimorphodon, one of the oldest known flying reptiles, a typical representative of this group.
Archaeopteryx, the oldest known bird.
The land reptile Euparkeria, claimed by many evolutionist authorities to be the ancestor of birds and flying reptiles.
The oldest known bird (Archaeopteryx), a flying reptile, and a land reptile that evolutionists claim to have been these creatures' closest ancestor. The differences between them are very great.
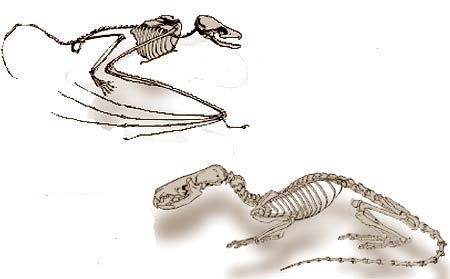
The skeleton of the oldest bat (Icaronycteris) from the Eocene.
The oldest known bat, and what evolutionists claim is its closest ancestor. Note the great difference between the bat and its so-called ancestor.
A modern shrew, which closely resembles the ancient insectivores claimed to be the ancestors of bats.
There is no end to such questions, and evolution has no answer to any of them. Instead, the theory of evolution makes do with an unbelievable story. Consider all the coincidences that this story involves in the case of marine mammals. First of all, fish just happened to come into existence in the water. Next, they made the transition to land by pure chance. Following this, they evolved on the land into reptiles and mammals, also by chance alone. Finally, it just so happened that some of these creatures returned to the water where by chance they acquired all the features they would need to survive there.
Can the theory of evolution prove even a single one of these stages? Certainly not. Far from being able to prove the claim as a whole, the theory of evolution is unable to demonstrate how even one of these different steps could have happened.

Skeleton of modern seal, virtually identical to the earliest known seals of the Miocene era.
Cynodictis gregarius, the land-dwelling carnivorous mammal which evolutionists believe to have been seals' closest ancestor.
A typical seal skeleton, and what evolutionists believe to be its nearest land-dwelling ancestor. Again, there is a huge difference between the two.

Halitherium, an early sea cow from the Oligocene
Hyrax, which is considered to be the nearest terrestrial ancestor of the sirenian aquatic mammals which also include sea cows.
A sea cow, and what evolutionists call its nearest terrestrial ancestor.
All the findings we have examined so far reveal that species appeared on earth suddenly and fully formed, with no evolutionary process prior to them. If this is so, then this is concrete evidence that living things are created, as evolutionary biologist Douglas Futuyma has acknowledged. Recall that he wrote:
"If they did appear in a fully developed state, they must indeed have been created by some omnipotent intelligence."172
Evolutionists, on the other hand, try to interpret the sequence by which living things appeared on earth as evidence for evolution. However, since no such evolutionary process ever took place, this sequence can only be the sequence of creation. Fossils reveal that living things appeared on earth first in the sea, and then on land, followed by the appearance of man.
106 John Ostrom, "Bird Flight: How Did It Begin?," American Scientist, January-February 1979, vol. 67, p. 47.
107 Robert L. Carroll, Patterns and Processes of Vertebrate Evolution, Cambridge University Press, 1997, p. 314.
108 Pat Shipman, "Birds Do It... Did Dinosaurs?," New Scientist, 1 February 1997, p. 28.
109 Pat Shipman, "Birds Do It... Did Dinosaurs?," New Scientist, 1 February 1997, p. 28.
110 Duane T. Gish, Dinosaurs by Design, Master Books, AR, 1996, pp. 65-66.
111 Michael Denton, A Theory in Crisis, Adler & Adler, 1986, pp. 210-211.
112 Michael Denton, A Theory in Crisis, Adler & Adler, 1986, pp. 211-212. (emphasis added)
113 J. A. Ruben, T. D. Jones, N. R. Geist, and W. J. Hillenius, "Lung Structure And Ventilation in Theropod Dinosaurs and Early Birds," Science, vol. 278, p. 1267.
114 Michael J. Denton, Nature's Destiny, Free Press, New York, 1998, p. 361.
115 Michael J. Denton, Nature's Destiny, Free Press, New York, 1998, pp. 361-62.
116 Barbara J. Stahl, Vertebrate History: Problems in Evolution, Dover, 1985, pp. 349-350. (emphasis added)
117 A. H. Brush, "On the Origin of Feathers," Journal of Evolutionary Biology, vol. 9, 1996, p.132.
118 A. H. Brush, "On the Origin of Feathers," Journal of Evolutionary Biology, vol. 9, 1996, p.131.
119 A. H. Brush, "On the Origin of Feathers," Journal of Evolutionary Biology, vol. 9, 1996, p.133.
120 A. H. Brush, "On the Origin of Feathers," Journal of Evolutionary Biology, vol. 9, 1996, p.131.
121 Alan Feduccia, "On Why Dinosaurs Lacked Feathers," The Beginning of Birds, Eichstatt, West Germany: Jura Museum, 1985, p. 76. (emphasis added)
122 Ernst Mayr, Systematics and the Origin of Species, Dover, New York, 1964, p. 296.
123 Norman Macbeth, Darwin Retried: An Appeal to Reason, Harvard Common Press, 1971, p. 131.
124 Nature, vol. 382, August, 1, 1996, p. 401.
125 Carl O. Dunbar, Historical Geology, John Wiley and Sons, New York, 1961, p. 310.
126 Robert L. Carroll, Patterns and Processes of Vertebrate Evolution, Cambridge University Press, 1997, p. 280-81.
127 L. D. Martin, J. D. Stewart, K. N. Whetstone, The Auk, vol. 97, 1980, p. 86.
128 L. D. Martin, J. D. Stewart, K. N. Whetstone, The Auk, vol. 97, 1980, p. 86; L. D. Martin, "Origins of the Higher Groups of Tetrapods", Ithaca, Comstock Publishing Association, New York, 1991, pp. 485-540.
129 S. Tarsitano, M. K. Hecht, Zoological Journal of the Linnaean Society, vol. 69, 1980, p. 149; A. D. Walker, Geological Magazine, vol. 117, 1980, p. 595.
130 A.D. Walker, as described in Peter Dodson, "International Archaeopteryx Conference," Journal of Vertebrate Paleontology 5(2):177, June 1985.
131 Richard Hinchliffe, "The Forward March of the Bird-Dinosaurs Halted?," Science, vol. 278, no. 5338, 24 October 1997, pp. 596-597.
132 Jonathan Wells, Icons of Evolution, Regnery Publishing, 2000, p. 117
133 Richard L. Deem, "Demise of the 'Birds are Dinosaurs' Theory,"
http://www.yfiles.com/dinobird2.html.
134 Pat Shipman, "Birds do it... Did Dinosaurs?," New Scientist, 1 February, 1997, p. 31.
135 "Old Bird," Discover, March 21, 1997.
136 "Old Bird," Discover, March 21, 1997.
137 Pat Shipman, "Birds Do It... Did Dinosaurs?," p. 28.
138 Ann Gibbons, "Plucking the Feathered Dinosaur," Science, vol. 278, no. 5341, 14 November 1997, pp. 1229 - 1230
139 National Geographic, Vol. 196, No. 5, November 1999, "Feathers for T. Rex?"
140 Tim Friend, "Dinosaur-bird link smashed in fossil flap," USA Today, 25 January 2000
141 "Open Letter: Smithsonian decries National Geographic's "editorial propagandizing" of dinosaur-to-bird "evolution," http://www.trueorigin.org/ birdevoletter.asp
142 M. Kusinitz, Science World, 4 February, 1983, p. 19.
143 San Diego Union, New York Times Press Service, 29 May, 1983; W. A. Shear, Science, vol. 224, 1984, p. 494. (emphasis added)
144 R. J. Wootton, C. P. Ellington, "Biomechanics & the Origin of Insect Flight," Biomechanics in Evolution, ed. J. M. V. Rayner & R. J. Wootton, Cambridge University Press, Cambridge, 1991, p. 99.
145 Robin J. Wootton, "The Mechanical Design of Insect Wings," Scientific American, vol. 263, November 1990, p. 120. (emphasis added)
146 Pierre-P Grassé, Evolution of Living Organisms, Academic Press, New York, 1977, p. 30. (emphasis added)
147 George Gamow, Martynas Ycas, Mr. Tompkins Inside Himself, The Viking Press, New York, 1967, p. 149.
148 Roger Lewin, "Bones of Mammals, Ancestors Fleshed Out," Science, vol. 212, June 26, 1981, p. 1492. (emphasis added)
149 George Gaylord Simpson, Life Before Man, Time-Life Books, New York, 1972, p. 42. (emphasis added)
150 R. Eric Lombard, "Review of Evolutionary Principles of the Mammalian Middle Ear, Gerald Fleischer," Evolution, vol. 33, December 1979, p. 1230.
151 George G., Simpson, Tempo and Mode in Evolution, Columbia University Press, New York, 1944, pp. 105, 107.
152 Boyce Rensberger, Houston Chronicle, November 5, 1980, p. 15. (emphasis added)
153 Niles Eldredge, quoted in Darwin's Enigma by Luther D. Sunderland, Santee, CA, Master Books, 1988, p. 78. (emphasis added)
154 Francis Hitching, The Neck of the Giraffe: Where Darwin Went Wrong, New American Library, New York, 1982, pp. 16-17, 19.
155 Francis Hitching, The Neck of the Giraffe: Where Darwin Went Wrong, New American Library, New York, 1982, pp. 16-17, 19.
156 Gordon Rattray Taylor, The Great Evolution Mystery, Abacus, Sphere Books, London, 1984, p. 230. (emphasis added)
157 John E. Hill, James D Smith, Bats: A Natural History, British Museum of Natural History, London, 1984, p. 33. (emphasis added)
158 L. R. Godfrey, "Creationism and Gaps in the Fossil Record," Scientists Confront Creationism, W. W. Norton and Company, 1983, p. 199.
159 Jeff Hecht, "Branching Out," New Scientist, 10 October 1998, vol. 160, no. 2155, p. 14.
160 Robert L. Carroll, Patterns and Processes of Vertebrate Evolution, Cambridge University Press, 1998, p.329.
161 Ashby L. Camp, "The Overselling of Whale Evolution," Creation Matters, a newsletter published by the Creation Research Society, May/June 1998.
162 Robert L. Carroll, Patterns and Processes of Vertebrate Evolution, Cambridge University Press, 1998, p. 333.
163 Douglas H. Chadwick, "Evolution of Whales," National Geographic, November 2001, p. 73.
164 Robert L. Carroll, Patterns and Processes of Vertebrate Evolution, Cambridge University Press, 1998, p. 329.
165 G. A. Mchedlidze, General Features of the Paleobiological Evolution of Cetacea, trans. from Russian (Rotterdam: A. A. Balkema, 1986), p. 91.
166 Douglas H. Chadwick, "Evolution of Whales," National Geographic, November 2001, p. 69.
167 Pierre-P Grassé, Evolution of Living Organisms, New York: Academic Press, 1977, p. 103.
168 B.J. Stahl, Vertebrate History: Problems in Evolution, Dover Publications Inc., 1985, p. 489.
169 Michel C. Milinkovitch, "Molecular phylogeny of cetaceans prompts revision of morphological transformations," Trends in Ecology and Evolution, 10 August 1995, pp. 328-334.
170 Uwe George, "Darwinismus der Irrtum des Jahrhunderts," Geo, January 1984, pp. 100-102.
171 Victor B. Scheffer, "Exploring the Lives of Whales," National Geographic, Vol. 50, December 1976, p. 752.
172 Douglas J. Futuyma, Science on Trial, Pantheon Books, New York, 1983, p. 197.