In previous sections of this book, we have shown how the fossil record invalidates the theory of evolution. In point of fact, there was no need for us to relate any of that, because the theory of evolution collapses long before one gets to any claims about the evidence of fossils. The subject that renders the theory meaningless from the very outset is the question of how life first appeared on earth.
When it addresses this question, evolutionary theory claims that life started with a cell that formed by chance. According to this scenario, four billion years ago various chemical compounds underwent a reaction in the primordial atmosphere on the earth in which the effects of thunderbolts and atmospheric pressure led to the formation of the first living cell.
The first thing that must be said is that the claim that nonliving materials can come together to form life is an unscientific one that has not been verified by any experiment or observation. Life is only generated from life. Each living cell is formed by the replication of another cell. No one in the world has ever succeeded in forming a living cell by bringing inanimate materials together, not even in the most advanced laboratories.
The theory of evolution claims that a living cell—which cannot be produced even when all the power of the human intellect, knowledge and technology are brought to bear—nevertheless managed to form by chance under primordial conditions on the earth. In the following pages, we will examine why this claim is contrary to the most basic principles of science and reason.
If one believes that a living cell can come into existence by chance, then there is nothing to prevent one from believing a similar story that we will relate below. It is the story of a town.
One day, a lump of clay, pressed between the rocks in a barren land, becomes wet after it rains. The wet clay dries and hardens when the sun rises, and takes on a stiff, resistant form. Afterwards, these rocks, which also served as a mould, are somehow smashed into pieces, and then a neat, well shaped, and strong brick appears. This brick waits under the same natural conditions for years for a similar brick to be formed. This goes on until hundreds and thousands of the same bricks have been formed in the same place. However, by chance, none of the bricks that were previously formed are damaged. Although exposed to storms, rain, wind, scorching sun, and freezing cold for thousands of years, the bricks do not crack, break up, or get dragged away, but wait there in the same place with the same determination for other bricks to form.
When the number of bricks is adequate, they erect a building by being arranged sideways and on top of each other, having been randomly dragged along by the effects of natural conditions such as winds, storms, or tornadoes. Meanwhile, materials such as cement or soil mixtures form under "natural conditions," with perfect timing, and creep between the bricks to clamp them to each other. While all this is happening, iron ore under the ground is shaped under "natural conditions" and lays the foundations of a building that is to be formed with these bricks. At the end of this process, a complete building rises with all its materials, carpentry, and installations intact.

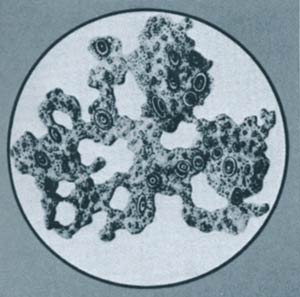
In Darwin's time, it was thought that the cell had a very simple structure. Darwin's ardent supporter Ernst Haeckel suggested that the above mud pulled up from the bottom of the sea could produce life by itself.
Of course, a building does not only consist of foundations, bricks, and cement. How, then, are the other missing materials to be obtained? The answer is simple: all kinds of materials that are needed for the construction of the building exist in the earth on which it is erected. Silicon for the glass, copper for the electric cables, iron for the columns, beams, water pipes, etc. all exist under the ground in abundant quantities. It takes only the skill of "natural conditions" to shape and place these materials inside the building. All the installations, carpentry, and accessories are placed among the bricks with the help of the blowing wind, rain, and earthquakes. Everything has gone so well that the bricks are arranged so as to leave the necessary window spaces as if they knew that something called glass would be formed later on by natural conditions. Moreover, they have not forgotten to leave some space to allow the installation of water, electricity and heating systems, which are also later to be formed by chance. Everything has gone so well that "coincidences" and "natural conditions" produce a perfect design.
One who manages to sustain his belief in this story so far should have no trouble surmising how the town's other buildings, plants, highways, sidewalks, substructures, communications, and transportation systems came about. If he possesses technical knowledge and is fairly conversant with the subject, he can even write an extremely "scientific" book of a few volumes stating his theories about "the evolutionary process of a sewage system and its uniformity with the present structures." He may well be honored with academic awards for his clever studies, and may consider himself a genius, shedding light on the nature of humanity.
The theory of evolution, which claims that life came into existence by chance, is no less absurd than our story, for, with all its operational systems, and systems of communication, transportation and management, a cell is no less complex than a city. In his book Evolution: A Theory in Crisis, the molecular biologist Michael Denton discusses the complex structure of the cell:
To grasp the reality of life as it has been revealed by molecular biology, we must magnify a cell a thousand million times until it is twenty kilometers in diameter and resembles a giant airship large enough to cover a great city like London or New York. What we would then see would be an object of unparalleled complexity and adaptive design. On the surface of the cell we would see millions of openings, like the port holes of a vast space ship, opening and closing to allow a continual stream of materials to flow in and out. If we were to enter one of these openings we would find ourselves in a world of supreme technology and bewildering complexity... Is it really credible that random processes could have constructed a reality, the smallest element of which—a functional protein or gene—is complex beyond our own creative capacities, a reality which is the very antithesis of chance, which excels in every sense anything produced by the intelligence of man?238

Fred Hoyle
The complex structure of the living cell was unknown in Darwin's day and at the time, ascribing life to "coincidences and natural conditions" was thought by evolutionists to be convincing enough. Darwin had proposed that the first cell could easily have formed "in some warm little pond."239 One of Darwin's supporters, the German biologist Ernst Haeckel, examined under the microscope a mixture of mud removed from the sea bed by a research ship and claimed that this was a nonliving substance that turned into a living one. This so-called "mud that comes to life," known as Bathybius haeckelii ("Haeckel's mud from the depths"), is an indication of just how simple a thing life was thought to be by the founders of the theory of evolution.
The technology of the twentieth century has delved into the tiniest particles of life, and has revealed that the cell is one of the most complex systems mankind has ever confronted. Today we know that the cell contains power stations producing the energy to be used by the cell, factories manufacturing the enzymes and hormones essential for life, a databank where all the necessary information about all products to be produced is recorded, complex transportation systems and pipelines for carrying raw materials and products from one place to another, advanced laboratories and refineries for breaking down external raw materials into their useable parts, and specialized cell membrane proteins to control the incoming and outgoing materials. And these constitute only a small part of this amazingly complex system.
W. H. Thorpe, an evolutionist scientist, acknowledges that "The most elementary type of cell constitutes a 'mechanism' unimaginably more complex than any machine yet thought up, let alone constructed, by man."240
A cell is so complex that even the high level of technology attained today cannot produce one. No effort to create an artificial cell has ever met with success. Indeed, all attempts to do so have been abandoned.
The theory of evolution claims that this system—which mankind, with all the intelligence, knowledge and technology at its disposal, cannot succeed in reproducing—came into existence "by chance" under the conditions of the primordial earth. Actually, the probability of forming a cell by chance is about the same as that of producing a perfect copy of a book following an explosion in a printing house.
The English mathematician and astronomer Sir Fred Hoyle made a similar comparison in an interview published in Nature magazine on November 12, 1981. Although an evolutionist himself, Hoyle stated that the probability that higher life forms might have emerged in this way is comparable to the probability that a tornado sweeping through a junk-yard might assemble a Boeing 747 from the materials therein.241 This means that it is not possible for the cell to have come into being by chance, and therefore it must definitely have been "created."
One of the basic reasons why the theory of evolution cannot explain how the cell came into existence is the "irreducible complexity" in it. A living cell maintains itself with the harmonious co-operation of many organelles. If only one of these organelles fails to function, the cell cannot remain alive. The cell does not have the opportunity to wait for unconscious mechanisms like natural selection or mutation to permit it to develop. Thus, the first cell on earth was necessarily a complete cell possessing all the required organelles and functions, and this definitely means that this cell had to have been created.
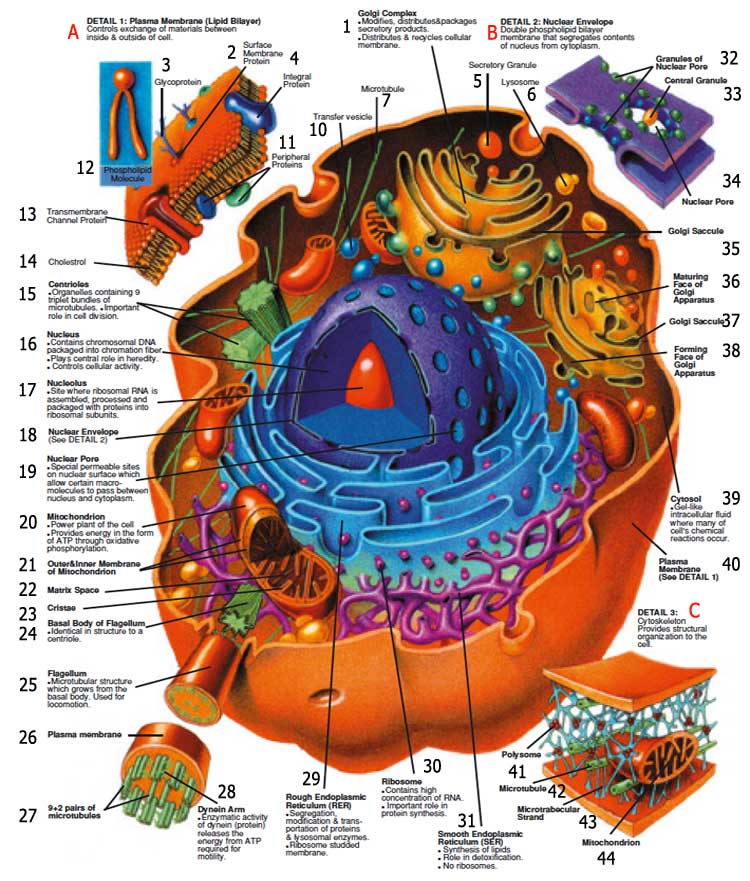 |
| A. DETAIL 1: Plasma Membrane (Lipid Bilayer); Controls exchange of materials between inside & outside of cell. |
| 1. Golgi Complex: Modifies, distributes&packages secretory products. Distributes & recycles cellular membrane. |
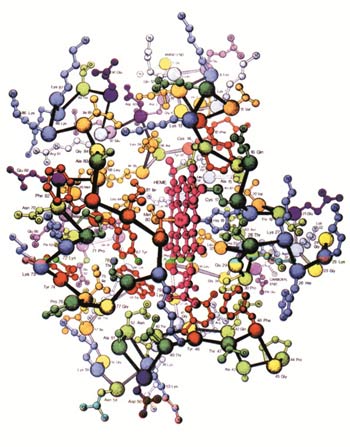
The complex 3-D structure of the protein cytochrome-C. The slightest difference in the order of the amino acids, represented by little balls, will render the protein nonfunctional.
So much for the cell, but evolution fails even to account for the building-blocks of a cell. The formation, under natural conditions, of just one single protein out of the thousands of complex protein molecules making up the cell is impossible.
Proteins are giant molecules consisting of smaller units called amino acids that are arranged in a particular sequence in certain quantities and structures. These units constitute the building blocks of a living protein. The simplest protein is composed of 50 amino acids, but there are some that contain thousands.
The absence, addition, or replacement of a single amino acid in the structure of a protein causes the protein to become a useless molecular heap. Every amino acid has to be in the right place and in the right order. The theory of evolution, which claims that life emerged as a result of chance, is quite helpless in the face of this order, since it is too wondrous to be explained by coincidence. Prof. Fred Hoyle comments as follows:
Indeed, such a theory (that life was assembled by an intelligence) is so obvious that one wonders why it is not widely accepted as being self-evident. The reasons are psychological rather than scientific.242
The reason Hoyle used the term "psychological" is the self-conditioning of evolutionists not to accept that life was created. The rejection of Allah's existence is their main goal. For this reason alone, they go on defending irrational theories which they at the same time acknowledge to be impossible.
Let us now examine in detail why the evolutionist scenario regarding the formation of proteins is impossible.
Even the correct sequence of the right amino acids is still not enough for the formation of a functional protein molecule. In addition to these requirements, each of the 20 different types of amino acids present in the composition of proteins must be left-handed. There are two different types of amino acids—as of all organic molecules—called "left-handed" and "right-handed." The difference between them is the mirror-symmetry between their three dimensional structures, which is similar to that of a person's right and left hands.
Amino acids of either of these two types can easily bond with one another. But one astonishing fact that has been revealed by research is that all the proteins in plants and animals on this planet, from the simplest organism to the most complex, are made up of left-handed amino acids. If even a single right-handed amino acid gets attached to the structure of a protein, the protein is rendered useless. In a series of experiments, surprisingly, bacteria that were exposed to right-handed amino acids immediately destroyed them. In some cases, they produced usable left-handed amino acids from the fractured components.
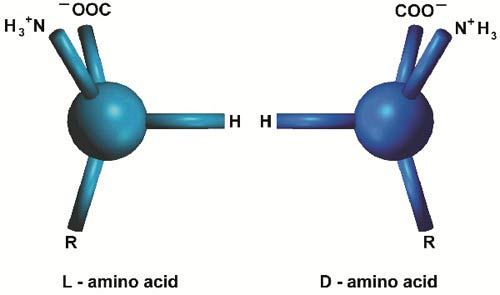
L - Left-handed amino acid
D - Right-handed amino acid
The same protein's left- (L) and right- (D) handed isomers. The proteins in living creatures consist only of left-handed amino acids.
Let us for an instant suppose that life came about by chance as evolutionists claim it did. In this case, the right- and left-handed amino acids that were generated by chance should be present in roughly equal proportions in nature. Therefore, all living things should have both right- and left-handed amino acids in their constitution, because chemically it is possible for amino acids of both types to combine with each other. However, as we know, in the real world the proteins existing in all living organisms are made up only of left-handed amino acids.
The question of how proteins can pick out only the left-handed ones from among all amino acids, and how not even a single right-handed amino acid gets involved in the life process, is a problem that still baffles evolutionists. Such a specific and conscious selection constitutes one of the greatest impasses facing the theory of evolution.
Moreover, this characteristic of proteins makes the problem facing evolutionists with respect to "chance" even worse. In order for a "meaningful" protein to be generated, it is not enough for the amino acids to be present in a particular number and sequence, and to be combined together in the right three-dimensional design. Additionally, all these amino acids have to be left-handed: not even one of them can be right-handed. Yet there is no natural selection mechanism which can identify that a right-handed amino acid has been added to the sequence and recognize that it must therefore be removed from the chain. This situation once more eliminates for good the possibility of coincidence and chance.
The Britannica Science Encyclopaedia, which is an outspoken defender of evolution, states that the amino acids of all living organisms on earth, and the building blocks of complex polymers such as proteins, have the same left-handed asymmetry. It adds that this is tantamount to tossing a coin a million times and always getting heads. The same encyclopaedia states that it is impossible to understand why molecules become left-handed or right-handed, and that this choice is fascinatingly related to the origin of life on earth.243
If a coin always turns up heads when tossed a million times, is it more logical to attribute that to chance, or else to accept that there is conscious intervention going on? The answer should be obvious. However, obvious though it may be, evolutionists still take refuge in coincidence, simply because they do not want to accept the existence of conscious intervention.
A situation similar to the left-handedness of amino acids also exists with respect to nucleotides, the smallest units of the nucleic acids, DNA and RNA. In contrast to proteins, in which only left-handed amino acids are chosen, in the case of the nucleic acids, the preferred forms of their nucleotide components are always right-handed. This is another fact that can never be explained by chance.
The difficulties the theory of evolution is unable to overcome with regard to the development of a single protein are not limited to those we have recounted so far. It is not enough for amino acids to be arranged in the correct numbers, sequences, and required three-dimensional structures. The formation of a protein also requires that amino acid molecules with more than one arm be linked to each other only in certain ways. Such a bond is called a "peptide bond." Amino acids can make different bonds with each other; but proteins are made up of those—and only those—amino acids which are joined by peptide bonds.
A comparison will clarify this point. Suppose that all the parts of a car were complete and correctly assembled, with the sole exception that one of the wheels was fastened in place not with the usual nuts and bolts, but with a piece of wire, in such a way that its hub faced the ground. It would be impossible for such a car to move even the shortest distance, no matter how complex its technology or how powerful its engine. At first glance, everything would seem to be in the right place, but the faulty attachment of even one wheel would make the entire car useless. In the same way, in a protein molecule the joining of even one amino acid to another with a bond other than a peptide bond would make the entire molecule useless.
Research has shown that amino acids combining at random combine with a peptide bond only 50 percent of the time, and that the rest of the time different bonds that are not present in proteins emerge. To function properly, each amino acid making up a protein must be joined to others only with a peptide bond, in the same way that it likewise must be chosen only from among left-handed forms.
Since some people are unable to take a broad view of these matters, but approach them from a superficial viewpoint and assume protein formation to be a simple chemical reaction, they may make unrealistic deductions such as "amino acids combine by way of reaction and then form proteins." However, accidental chemical reactions taking place in a nonliving structure can only bring about simple compounds. The number of these is predetermined and limited. For a somewhat more complex chemical material, huge factories, chemical plants, and laboratories have to be involved. Medicines and many other chemical materials that we use in our daily life are made in just this way. Proteins have much more complex structures than these chemicals produced by industry. Therefore, it is impossible for proteins, each of which is a wonder of creation and engineering, in which every part takes its place in a fixed order, to originate as a result of haphazard chemical reactions.
To summarize the subject of proteins;
• Around 100 special proteins are needed for a single protein to form.
• Protein cannot form if even one of these enzymes (proteins) required for protein synthesis is missing.
• It is not enough for these 100 enzymes to be present at the same time; they must all also be present in a special region inside the cell (a specific region inside the nucleus).
• DNA manufactures the enzymes necessary for protein to form. Proteins are also needed for DNA replication. There is no possibility of one appearing before the other. Both have to be present at the same time.
• A ribosome that serves as a factory for protein formation must also exist. But the ribosome is itself made up of proteins. Therefore, proteins are needed for ribosomes to exist, and ribosomes are needed for proteins.
• It is impossible for one to form before the other. Proteins, DNA, the ribosome, the cell nucleus, mitochondria that produce energy and all the other organelles in the cell must all exist at one and the same time.
• The enzymes essential for protein to form have to be sent to the region where manufacture will be carried out by the cell. Even if enzymes are present, so long as they are not given tasks to perform by the cell they will do nothing for that protein.
• There have to be a specific temperature and pH value in order for enzymes to be able to carry out reactions. Enzymes do not initiate reactions if they are not at the right temperature and pH level.
• Therefore, it is impossible for a protein to emerge so long as all the organelles of the cell do not co-exist together.
• Even if we place all the components necessary for protein in some muddy water, these components can never combine together to constitute proteins. The existence of the cell is a prerequisite for that to happen.
• Amino acids do not normally react with one another. Helper enzymes to carry out a reaction have to be ready and present inside the cell. But they do naturally enter into reactions with various substances, such as sugar. Therefore, even if all the requisite amino acids are placed into muddy water they can still never combine spontaneously with other amino acids. The cell is again essential for that to happen.
• Under natural conditions, even if a protein is left inside muddy water, that protein will immediately be broken down, under the effect of various environmental factors, or else will combine with other acids, amino acids or chemical substances and lose all its properties and turn into another substance that serves no purpose.
• In addition to all this, it will be useful to reiterate the essential conditions for a protein:
a. There must be peptide bonds between amino acids
b. All amino acids must be left-handed
c. Only 20 amino acids must be used
d. Amino acids have to be in a specific sequence
e. The protein that forms has to have a specific 3-D shape.
Let us for a minute put aside all the impossibilities we have described so far, and suppose that a useful protein molecule still evolved spontaneously "by accident." Even so, the theory of evolution again has no answers, because in order for this protein to survive, it would need to be isolated from its natural habitat and be protected under very special conditions. Otherwise, it would either disintegrate from exposure to natural conditions on earth, or else join with other acids, amino acids, or chemical compounds, thereby losing its particular properties and turning into a totally different and useless substance.
What we have been discussing so far is the impossibility of just one protein's coming about by chance. However, in the human body alone there are some 100,000 proteins functioning. Furthermore, there are about 1.5 million species named, and another 10 million are believed to exist. Although many similar proteins are used in many life forms, it is estimated that there must be 100 million or more types of protein in the plant and animal worlds. And the millions of species which have already become extinct are not included in this calculation. In other words, hundreds of millions of protein codes have existed in the world. If one considers that not even one protein can be explained by chance, it is clear what the existence of hundreds of millions of different proteins must mean.
Bearing this truth in mind, it can clearly be understood that "coincidences" cannot account for the origin of living things.

1. Amino Acids
2. Transfer RNA
3. Codon
4. Messenger RNA
5 Ribosome
6. Protein Sequence
a. Val Valine
b. Cys Cysteine
c. Ala Alanine
Protein Synthesis:
The ribosome reads the messenger RNA, and arranges the amino acids according to the information it receives there. In the illustrations, the consecutive order of the [ val, cys, and ala amino acids ], established by the ribosome and transfer RNA, can be seen. All proteins in nature are produced by this complex process. No protein comes about by "accident."
Above all, there is one important point to take into consideration: If any one step in the evolutionary process is proven to be impossible, this is sufficient to prove that the whole theory is totally false and invalid. For instance, by proving that the haphazard formation of proteins is impossible, all other claims regarding the subsequent steps of evolution are also refuted. After this, it becomes meaningless to take some human and ape skulls and engage in speculation about them.
How living organisms came into existence out of nonliving matter was an issue that evolutionists did not even want to mention for a long time. However, this question, which had constantly been avoided, eventually had to be addressed, and attempts were made to settle it with a series of experiments in the second quarter of the twentieth century.
The main question was: How could the first living cell have appeared in the primordial atmosphere on the earth? In other words, what kind of explanation could evolutionists offer?
The first person to take the matter in hand was the Russian biologist Alexander I. Oparin, the founder of the concept of "chemical evolution." Despite all his theoretical studies, Oparin was unable to produce any results to shed light on the origin of life. He says the following in his book The Origin of Life, published in 1936:
Unfortunately, however, the problem of the origin of the cell is perhaps the most obscure point in the whole study of the evolution of organisms.244
Since Oparin, evolutionists have performed countless experiments, conducted research, and made observations to prove that a cell could have been formed by chance. However, every such attempt only made the complex structure of the cell clearer, and thus refuted the evolutionists' hypotheses even more. Professor Klaus Dose, the president of the Institute of Biochemistry at the University of Johannes Gutenberg, states:
More than 30 years of experimentation on the origin of life in the fields of chemical and molecular evolution have led to a better perception of the immensity of the problem of the origin of life on earth rather than to its solution. At present all discussions on principal theories and experiments in the field either end in stalemate or in a confession of ignorance.245
In his book The End of Science, the evolutionary science writer John Horgan says of the origin of life, "This is by far the weakest strut of the chassis of modern biology."246
The following statement by the geochemist Jeffrey Bada, from the San Diego-based Scripps Institute, makes the helplessness of evolutionists clear:
Today, as we leave the twentieth century, we still face the biggest unsolved problem that we had when we entered the twentieth century: How did life originate on Earth? 247
Let us now look at the details of the theory of evolution's "biggest unsolved problem". The first subject we have to consider is the famous Miller experiment.

Stanley Miller with his experimental apparatus.
The most generally respected study on the origin of life is the Miller experiment conducted by the American researcher Stanley Miller in 1953. (The experiment is also known as the "Urey-Miller experiment" because of the contribution of Miller's instructor at the University of Chicago, Harold Urey.) This experiment is the only "evidence" evolutionists have with which to allegedly prove the "chemical evolution thesis"; they advance it as the first stage of the supposed evolutionary process leading to life. Although nearly half a century has passed, and great technological advances have been made, nobody has made any further progress. In spite of this, Miller's experiment is still taught in textbooks as the evolutionary explanation of the earliest generation of living things. That is because, aware of the fact that such studies do not support, but rather actually refute, their thesis, evolutionist researchers deliberately avoid embarking on such experiments.
Stanley Miller's aim was to demonstrate by means of an experiment that amino acids, the building blocks of proteins, could have come into existence "by chance" on the lifeless earth billions of years ago. In his experiment, Miller used a gas mixture that he assumed to have existed on the primordial earth (but which later proved unrealistic), composed of ammonia, methane, hydrogen, and water vapor. Since these gases would not react with each other under natural conditions, he added energy to the mixture to start a reaction among them. Supposing that this energy could have come from lightning in the primordial atmosphere, he used an electric current for this purpose.
Miller heated this gas mixture at 100°C for a week and added the electrical current. At the end of the week, Miller analyzed the chemicals which had formed at the bottom of the jar, and observed that three out of the 20 amino acids which constitute the basic elements of proteins had been synthesized.
This experiment aroused great excitement among evolutionists, and was promoted as an outstanding success. Moreover, in a state of intoxicated euphoria, various publications carried headlines such as "Miller creates life." However, what Miller had managed to synthesize was only a few inanimate molecules.
Encouraged by this experiment, evolutionists immediately produced new scenarios. Stages following the development of amino acids were hurriedly hypothesized. Supposedly, amino acids had later united in the correct sequences by accident to form proteins. Some of these proteins which emerged by chance formed themselves into cell membrane–like structures which "somehow" came into existence and formed a primitive cell. These cells then supposedly came together over time to form multicellular living organisms.
However, Miller's experiment has since proven to be false in many respects.

The artificial atmosphere created by Miller in his experiment actually bore not the slightest resemblance to the primitive atmosphere on earth.
Miller's experiment sought to prove that amino acids could form on their own in primordial earth-like conditions, but it contains inconsistencies in a number of areas:
1- By using a mechanism called a "cold trap," Miller isolated the amino acids from the environment as soon as they were formed. Had he not done so, the conditions in the environment in which the amino acids were formed would immediately have destroyed these molecules.
Doubtless, this kind of conscious isolation mechanism did not exist on the primordial earth. Without such a mechanism, even if one amino acid were obtained, it would immediately have been destroyed. The chemist Richard Bliss expresses this contradiction by observing that "Actually, without this trap, the chemical products, would have been destroyed by the energy source."248 And, sure enough, in his previous experiments, Miller had been unable to make even one single amino acid using the same materials without the cold trap mechanism.
2- The primordial atmosphere that Miller attempted to simulate in his experiment was not realistic. In the 1980s, scientists agreed that nitrogen and carbon dioxide should have been used in this artificial environment instead of methane and ammonia.
So why did Miller insist on these gases? The answer is simple: without ammonia, it was impossible to synthesize any amino acid. Kevin Mc Kean talks about this in an article published in Discover magazine:
Miller and Urey imitated the ancient atmosphere on the Earth with a mixture of methane and ammonia. ...However in the latest studies, it has been understood that the Earth was very hot at those times, and that it was composed of melted nickel and iron. Therefore, the chemical atmosphere of that time should have been formed mostly of nitrogen (N2), carbon dioxide (CO2) and water vapour (H2O). However these are not as appropriate as methane and ammonia for the production of organic molecules.249
The American scientists J. P. Ferris and C. T. Chen repeated Miller's experiment with an atmospheric environment that contained carbon dioxide, hydrogen, nitrogen, and water vapor, and were unable to obtain even a single amino acid molecule. 250
3- Another important point that invalidates Miller's experiment is that there was enough oxygen to destroy all the amino acids in the atmosphere at the time when they were thought to have been formed. This fact, overlooked by Miller, is revealed by the traces of oxidized iron found in rocks that are estimated to be 3.5 billion years old.251

Today, Miller too accepts that his 1953 experiment was very far from explaining the origin of life.
There are other findings showing that the amount of oxygen in the atmosphere at that time was much higher than originally claimed by evolutionists. Studies also show that the amount of ultraviolet radiation to which the earth was then exposed was 10,000 times more than evolutionists' estimates. This intense radiation would unavoidably have freed oxygen by decomposing the water vapor and carbon dioxide in the atmosphere.
This situation completely negates Miller's experiment, in which oxygen was completely neglected. If oxygen had been used in the experiment, methane would have decomposed into carbon dioxide and water, and ammonia into nitrogen and water. On the other hand, in an environment where there was no oxygen, there would be no ozone layer either; therefore, the amino acids would have immediately been destroyed, since they would have been exposed to the most intense ultraviolet rays without the protection of the ozone layer. In other words, with or without oxygen in the primordial world, the result would have been a deadly environment for the amino acids.
4- At the end of Miller's experiment, many organic acids had also been formed with characteristics detrimental to the structure and function of living things. If the amino acids had not been isolated, and had been left in the same environment with these chemicals, their destruction or transformation into different compounds through chemical reactions would have been unavoidable.
Moreover, Miller's experiment also produced right-handed amino acids.252 The existence of these amino acids refuted the theory even within its own terms, because right-handed amino acids cannot function in the composition of living organisms. To conclude, the circumstances in which amino acids were formed in Miller's experiment were not suitable for life. In truth, this medium took the form of an acidic mixture destroying and oxidizing the useful molecules obtained.
All these facts point to one firm truth: Miller's experiment cannot claim to have proved that living things formed by chance under primordial earth–like conditions. The whole experiment is nothing more than a deliberate and controlled laboratory experiment to synthesize amino acids. The amount and types of the gases used in the experiment were ideally determined to allow amino acids to originate. The amount of energy supplied to the system was neither too much nor too little, but arranged precisely to enable the necessary reactions to occur. The experimental apparatus was isolated, so that it would not allow the leaking of any harmful, destructive, or any other kind of elements to hinder the formation of amino acids. No elements, minerals or compounds that were likely to have been present on the primordial earth, but which would have changed the course of the reactions, were included in the experiment. Oxygen, which would have prevented the formation of amino acids because of oxidation, is only one of these destructive elements. Even under such ideal laboratory conditions, it was impossible for the amino acids produced to survive and avoid destruction without the "cold trap" mechanism.
In fact, by his experiment, Miller destroyed evolution's claim that "life emerged as the result of unconscious coincidences." That is because, if the experiment proves anything, it is that amino acids can only be produced in a controlled laboratory environment where all the conditions are specifically designed by conscious intervention.
Today, Miller's experiment is totally disregarded even by evolutionist scientists. In the February 1998 issue of the famous evolutionist science journal Earth, the following statements appear in an article titled "Life's Crucible":
Geologist now think that the primordial atmosphere consisted mainly of carbon dioxide and nitrogen, gases that are less reactive than those used in the 1953 experiment. And even if Miller's atmosphere could have existed, how do you get simple molecules such as amino acids to go through the necessary chemical changes that will convert them into more complicated compounds, or polymers, such as proteins? Miller himself throws up his hands at that part of the puzzle. "It's a problem," he sighs with exasperation. "How do you make polymers? That's not so easy."253
As seen, today even Miller himself has accepted that his experiment does not lead to an explanation of the origin of life. In the March 1998 issue of National Geographic, in an article titled "The Emergence of Life on Earth," the following comments appear:
Many scientists now suspect that the early atmosphere was different to what Miller first supposed. They think it consisted of carbon dioxide and nitrogen rather than hydrogen, methane, and ammonia.
That's bad news for chemists. When they try sparking carbon dioxide and nitrogen, they get a paltry amount of organic molecules - the equivalent of dissolving a drop of food colouring in a swimming pool of water. Scientists find it hard to imagine life emerging from such a diluted soup.254
In brief, neither Miller's experiment, nor any other similar one that has been attempted, can answer the question of how life emerged on earth. All of the research that has been done shows that it is impossible for life to emerge by chance, and thus confirms that life is created. The reason evolutionists do not accept this obvious reality is their blind adherence to prejudices that are totally unscientific. Interestingly enough, Harold Urey, who organized the Miller experiment with his student Stanley Miller, made the following confession on this subject:
All of us who study the origin of life find that the more we look into it, the more we feel it is too complex to have evolved anywhere. We all believe as an article of faith that life evolved from dead matter on this planet. It is just that its complexity is so great, it is hard for us to imagine that it did.255
Evolutionist sources use the Miller experiment, despite all of its inconsistencies, to try to gloss over the question of the origin of amino acids. By giving the impression that the issue has long since been resolved by that invalid experiment, they try to paper over the cracks in the theory of evolution.
However, to explain the second stage of the origin of life, evolutionists faced an even greater problem than that of the formation of amino acids—namely, the origin of proteins, the building blocks of life, which are composed of hundreds of different amino acids bonding with each other in a particular order.
Claiming that proteins were formed by chance under natural conditions is even more unrealistic and unreasonable than claiming that amino acids were formed by chance. In the preceding pages we have already seen the impossibility of the haphazard formation of proteins. Now, we will further examine the impossibility of proteins being produced chemically under primordial earth conditions.
As we saw before, when combining to form proteins, amino acids form a special bond with one another called the peptide bond. A water molecule is released during the formation of this peptide bond.
This fact definitely refutes the evolutionist explanation that primordial life originated in water, because, according to the "Le Châtelier principle" in chemistry, it is not possible for a reaction that releases water (a condensation reaction) to take place in a hydrous environment. The probability of this kind of a reaction happening in a hydrate environment is said to "have the least probability of occurring" of all chemical reactions.
Hence the ocean, which is claimed to be where life began and amino acids originated, is definitely not an appropriate setting for amino acids to form proteins.256 On the other hand, it would be irrational for evolutionists to change their minds and claim that life originated on land, because the only environment where amino acids could have been protected from ultraviolet radiation is in the oceans and seas. On land, they would be destroyed by ultraviolet rays. The Le Châtelier principle, on the other hand, disproves the claim of the formation of life in the sea. This is another dilemma confronting evolution.
Challenged by the abovementioned dilemma, evolutionists began to invent unrealistic scenarios based on this "water problem" that so definitively refuted their theories. Sydney Fox was one of the best known of these researchers. Fox advanced the following theory to solve the problem. According to him, the first amino acids must have been transported to some cliffs near a volcano right after their formation in the primordial ocean. The water contained in this mixture that included the amino acids must have evaporated when the temperature increased above boiling point on the cliffs. The amino acids which were "dried out" in this way, could then have combined to form proteins.
However this "complicated" way out was not accepted by many people in the field, because the amino acids could not have endured such high temperatures. Research confirmed that amino acids are immediately destroyed at very high temperatures.
But Fox did not give up. He combined purified amino acids in the laboratory, "under very special conditions," by heating them in a dry environment. The amino acids combined, but still no proteins were obtained. What he actually ended up with was simple and disordered loops of amino acids, arbitrarily combined with each other, and these loops were far from resembling any living protein. Furthermore, if Fox had kept the amino acids at a steady temperature, then these useless loops would also have disintegrated.
Another point that nullified the experiment was that Fox did not use the useless end products obtained in Miller's experiment; rather, he used pure amino acids from living organisms. This experiment, however, which was intended to be a continuation of Miller's experiment, should have started out from the results obtained by Miller. Yet neither Fox, nor any other researcher, used the useless amino acids Miller produced.
Fox's experiment was not even welcomed in evolutionist circles, because it was clear that the meaningless amino acid chains that he obtained (which he termed "proteinoids") could not have formed under natural conditions. Moreover, proteins, the basic units of life, still could not be produced. The problem of the origin of proteins remained unsolved. In an article in the popular science magazine, Chemical Engineering News, which appeared in the 1970s, Fox's experiment was mentioned as follows:
Sydney Fox and the other researchers managed to unite the amino acids in the shape of "proteinoids" by using very special heating techniques under conditions which in fact did not exist at all in the primordial stages of Earth. Also, they are not at all similar to the very regular proteins present in living things. They are nothing but useless, irregular chemical stains. It was explained that even if such molecules had formed in the early ages, they would definitely be destroyed.257
Indeed, the proteinoids Fox obtained were totally different from real proteins, both in structure and function. The difference between proteins and these proteinoids was as huge as the difference between a piece of high-tech equipment and a heap of unprocessed iron.
Furthermore, it was impossible for these irregular amino acid chains to have survived in the primordial atmosphere. Harmful and destructive physical and chemical effects caused by heavy exposure to ultraviolet light and other unstable natural conditions would have caused these proteinoids to disintegrate. Because of the Le Châtelier principle, it was also impossible for the amino acids to combine underwater, where ultraviolet rays would not reach them. In view of this, the idea that the proteinoids were the basis of life eventually lost support among scientists.
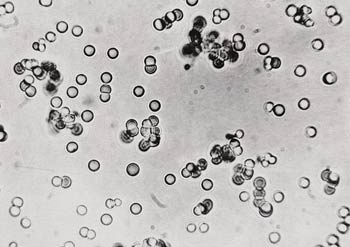
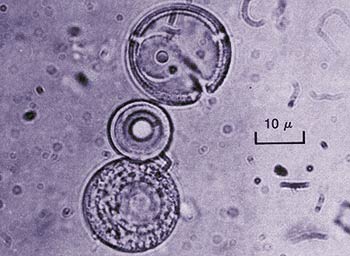
Fox's "Proteinoids"
Sydney Fox, who was influenced by Miller's scenario, formed the above molecules, which he called "proteinoids," by joining amino acids together. However, these chains of nonfunctioning amino acids had no resemblance to the real proteins that make up the bodies of living things. Actually, all these efforts showed not only that life did not come about by chance, but also that it could not be reproduced in laboratory conditions.
Our examinations so far have shown that the theory of evolution is in a serious quandary at the molecular level. Evolutionists have shed no light on the formation of amino acids at all. The formation of proteins, on the other hand, is another mystery all its own.
Yet the problems are not even limited just to amino acids and proteins: These are only the beginning. Beyond them, the extremely complex structure of the cell leads evolutionists to yet another impasse. The reason for this is that the cell is not just a heap of amino-acid-structured proteins, but rather one of the most complex systems man has ever encountered.
While the theory of evolution was having such trouble providing a coherent explanation for the existence of the molecules that are the basis of the cell structure, developments in the science of genetics and the discovery of nucleic acids (DNA and RNA) produced brand-new problems for the theory. In 1953, James Watson and Francis Crick launched a new age in biology with their work on the structure of DNA.
The molecule known as DNA, which is found in the nucleus of each of the 100 trillion cells in our bodies, contains the complete blueprint for the construction of the human body. The information regarding all the characteristics of a person, from physical appearance to the structure of the inner organs, is recorded in DNA within the sequence of four special bases that make up the giant molecule. These bases are known as A, T, G, and C, according to the initial letters of their names. All the structural differences among people depend on variations in the sequences of these letters. In addition to features such as height, and eye, hair and skin colors, the DNA in a single cell also contains the design of the 206 bones, the 600 muscles, the 100 billion nerve cells (neurons), 1.000 trillion connections between the neurons of the brain, 97,000 kilometers of veins, and the 100 trillion cells of the human body. If we were to write down the information coded in DNA, then we would have to compile a giant library consisting of 900 volumes of 500 pages each. But the information this enormous library would hold is encoded inside the DNA molecules in the cell nucleus, which is far smaller than the 1/100th-of-a-millimeter-long cell itself.

When Watson and Crick discovered the structure of DNA, they revealed that life was much more complicated than had previously been thought.
At this point, there is an important detail that deserves attention. An error in the sequence of the nucleotides making up a gene would render that gene completely useless. When it is considered that there are some 30,000 genes in the human body, it becomes clearer how impossible it is for the millions of nucleotides making up these genes to have been formed, in the right sequence, by chance.
The impossibility of the formation of RNA and DNA by a coincidental accumulation of nucleotides is expressed by the French scientist Paul Auger in this way:
We have to sharply distinguish the two stages in the chance formation of complex molecules such as nucleotides by chemical events. The production of nucleotides one by one—which is possible—and the combination of these within very special sequences. The second is absolutely impossible.258
For many years, Francis Crick believed in the theory of molecular evolution, but eventually even he had to admit to himself that such a complex molecule could not have emerged spontaneously by chance, as the result of an evolutionary process:
An honest man, armed with all the knowledge available to us now, could only state that, in some sense, the origin of life appears at the moment to be almost a miracle.259
The Turkish evolutionist Professor Ali Demirsoy was forced to make the following confession on the issue:
In fact, the probability of the formation of a protein and a nucleic acid (DNA-RNA) is a probability way beyond estimating. Furthermore, the probability of the emergence of a certain protein chain is so slight as to be called astronomic.260

DNA codes of the beta-globin gene. These codes make up one of the parts of the haemoglobin that carry oxygen in the blood. The important thing is that if there is an error in just one of these codes, the protein that is produced will be totally useless.
A very interesting paradox emerges at this point: While DNA can only replicate with the help of special proteins (enzymes), the synthesis of these proteins can only be realized by the information encoded in DNA. As they both depend on each other, they have to exist at the same time for replication. Science writer John Horgan explains the dilemma in this way:
DNA cannot do its work, including forming more DNA, without the help of catalyticproteins, or enzymes. In short, proteins cannot form without DNA, but neither can DNA form without proteins. 261
This situation once again undermines the scenario that life could have come about by accident. Homer Jacobson, Professor Emeritus of Chemistry, comments:
Directions for the reproduction of plans, for energy and the extraction of parts from the current environment, for the growth sequence, and for the effector mechanism translating instructions into growth—all had to be simultaneously present at that moment [when life began]. This combination of events has seemed an incredibly unlikely happenstance...262
The quotation above was written two years after the discovery of the structure of DNA by Watson and Crick. But despite all the developments in science, this problem for evolutionists remains unsolved. This is why German biochemist Douglas R. Hofstadter says:
'How did the Genetic Code, along with the mechanisms for its translation (ribosomes and RNA molecules), originate?' For the moment, we will have to content ourselves with a sense of wonder and awe, rather than with an answer.263
Stanley Miller and Francis Crick's close associate from the University of San Diego, California, the highly reputed evolutionist Dr. Leslie Orgel says in an article published in 1994:
It is extremely improbable that proteins and nucleic acids, both of which are structurally complex, arose spontaneously in the same place at the same time. Yet it also seems impossible to have one without the other. And so, at first glance, one might have to conclude that life could never, in fact, have originated by chemical means.264
Alongside all of this, it is chemically impossible for nucleic acids such as DNA and RNA, which possess a definite string of information, to have emerged by chance, or for even one of the nucleotides which compose them to have come about by accident and to have survived and maintained its unadulterated state under the conditions of the primordial world. Even the famous journal Scientific American, which follows an evolutionist line, has been obliged to confess the doubts of evolutionists on this subject:
Even the simpler molecules are produced only in small amounts in realistic experiments simulating possible primitive earth conditions. What is worse, these molecules are generally minor constituents of tars: It remains problematical how they could have been separated and purified through geochemical processes whose normal effects are to make organic mixtures more and more of a jumble. With somewhat more complex molecules these difficulties rapidly increase. In particular a purely geochemical origin of nucleotides (the subunits of DNA and RNA) presents great difficulties.265
As revealed by what has been discussed so far, since it is impossible for life to have emerged by chemical means, life was created by All Powerful Allah. This "chemical evolution" that evolutionists have been talking about since the beginning of the last century never happened, and is nothing but a myth.
But most evolutionists believe in this and similar totally unscientific fairy tales as if they were true, because accepting that living things were created means accepting Almighty Allah's existence—and they have conditioned themselves not to accept this truth. One famous biologist from Australia, Michael Denton, discusses the subject in his book Evolution: A Theory in Crisis:
To the skeptic, the proposition that the genetic programmes of higher organisms, consisting of something close to a thousand million bits of information, equivalent to the sequence of letters in a small library of 1,000 volumes, containing in encoded form countless thousands of intricate algorithms controlling, specifying, and ordering the growth and development of billions and billions of cells into the form of a complex organism, were composed by a purely random process is simply an affront to reason. But to the Darwinist, the idea is accepted without a ripple of doubt - the paradigm takes precedence!266

The extraordinary information concealed in DNA is clear proof that life did not emerge by chance, but was deliberately created. No natural process can account for the origin of DNA.
The discovery in the 1970s that the gases originally existing in the primitive atmosphere of the earth would have rendered amino acid synthesis impossible was a serious blow to the theory of molecular evolution. Evolutionists then had to face the fact that the "primitive atmosphere experiments" by Stanley Miller, Sydney Fox, Cyril Ponnamperuma and others were invalid. For this reason, in the 1980s the evolutionists tried again. As a result, the "RNA World" hypothesis was advanced. This scenario proposed that, not proteins, but rather the RNA molecules that contained the information for proteins, were formed first.
According to this scenario, advanced by Harvard chemist Walter Gilbert in 1986, inspired by the discovery about "ribozymes" by Thomas Cech, billions of years ago an RNA molecule capable of replicating itself formed somehow by accident. Then this RNA molecule started to produce proteins, having been activated by external influences. Thereafter, it became necessary to store this information in a second molecule, and somehow the DNA molecule emerged to do that.
Made up as it is of a chain of impossibilities in each and every stage, this scarcely credible scenario, far from providing any explanation of the origin of life, only magnified the problem, and raised many unanswerable questions:
1. Since it is impossible to accept the coincidental formation of even one of the nucleotides making up RNA, how can it be possible for these imaginary nucleotides to form RNA by coming together in a particular sequence? Evolutionist John Horgan admits the impossibility of the chance formation of RNA;
As researchers continue to examine the RNA-World concept closely, more problems emerge. How did RNA initially arise? RNA and its components are difficult to synthesize in a laboratory under the best of conditions, much less under really plausible ones.267
2. Even if we suppose that it formed by chance, how could this RNA, consisting of just a nucleotide chain, have "decided" to self-replicate, and with what kind of mechanism could it have carried out this self-replicating process? Where did it find the nucleotides it used while self-replicating? Even evolutionist microbiologists Gerald Joyce and Leslie Orgel express the desperate nature of the situtation in their book In the RNA World:
This discussion… has, in a sense, focused on a straw man: the myth of a self-replicating RNA molecule that arose de novo from a soup of random polynucleotides. Not only is such a notion unrealistic in light of our current understanding of prebiotic chemistry, but it would strain the credulity of even an optimist's view of RNA's catalytic potential.268
3. Even if we suppose that there was self-replicating RNA in the primordial world, that numerous amino acids of every type ready to be used by RNA were available, and that all of these impossibilities somehow took place, the situation still does not lead to the formation of even one single protein. For RNA only includes information concerning the structure of proteins. Amino acids, on the other hand, are raw materials. Nevertheless, there is no mechanism for the production of proteins. To consider the existence of RNA sufficient for protein production is as nonsensical as expecting a car to assemble itself by simply throwing the blueprint onto a heap of parts piled up on top of each other. A blueprint cannot produce a car all by itself without a factory and workers to assemble the parts according to the instructions contained in the blueprint; in the same way, the blueprint contained in RNA cannot produce proteins by itself without the cooperation of other cellular components which follow the instructions contained in the RNA.
Proteins are produced in the ribosome factory with the help of many enzymes, and as a result of extremely complex processes within the cell. The ribosome is a complex cell organelle made up of proteins. This leads, therefore, to another unreasonable supposition—that ribosomes, too, should have come into existence by chance at the same time. Even Nobel Prize winner Jacques Monod, who was one of the most fanatical defenders of evolution—and atheism—explained that protein synthesis can by no means be considered to depend merely on the information in the nucleic acids:
The code is meaningless unless translated. The modern cell's translating machinery consists of at least 50 macromolecular components, which are themselves coded in DNA: the code cannot be translated otherwise than by products of translation themselves. It is the modern expression of omne vivum ex ovo. When and how did this circle become closed? It is exceedingly difficult to imagine.269
How could an RNA chain in the primordial world have taken such a decision, and what methods could it have employed to make protein production happen by doing the work of 50 specialized particles on its own? Evolutionists have no answer to these questions. One article in the preeminent scientific journal Nature makes it clear that the concept of "self-replicating RNA" is a complete product of fantasy, and that actually this kind of RNA has not been produced in any experiment:
DNA replication is so error-prone that it needs the prior existence of protein enzymes to improve the copying fidelity of a gene-size piece of DNA. "Catch-22" say Maynard Smith and Szathmary. So, wheel on RNA with its now recognized properties of carrying both informational and enzymatic activity, leading the authors to state: "In essence, the first RNA molecules did not need a protein polymerase to replicate them; they replicated themselves." Is this a fact or a hope? I would have thought it relevant to point out for 'biologists in general' that not one self-replicating RNA has emerged to date from quadrillions (1024) of artificially synthesized, random RNA sequences.270
Dr. Leslie Orgel uses the term "scenario" for the possibility of "the origination of life through the RNA World." Orgel described what kind of features this RNA would have had to have and how impossible these would have been in his article "The Origin of Life," published in Scientific American in October 1994:
This scenario could have occurred, we noted, if prebiotic RNA had two properties not evident today: A capacity to replicate without the help of proteins and an ability to catalyze every step of protein synthesis.271
As should by now be clear, to expect these two complex and extremely essential processes from a molecule such as RNA is againt scientific thought. Concrete scientific facts, on the other hand, makes it explicit that the RNA World hypothesis, which is a new model proposed for the chance formation of life, is an equally implausible fable.
John Horgan, in his book The End of Science, reports that Stanley Miller viewed the theories subsequently put forward regarding the origin of life as quite meaningless (It will be recalled that Miller was the originator of the famous Miller Experiment, which was later revealed to be invalid.):
In fact, almost 40 years after his original experiment, Miller told me that solving the riddle of the origin of life had turned out to be more difficult than he or anyone else had envisioned… Miller seemed unimpressed with any of the current proposals on the origin of life, referring to them as "nonsense" or "paper chemistry." He was so contemptuous of some hypotheses that, when I asked his opinion of them, he merely shook his head, sighed deeply, and snickered—as if overcome by the folly of humanity. Stuart Kauffman's theory of autocatalysis fell into this category. "Running equations through a computer does not constitute an experiment," Miller sniffed. Miller acknowledged that scientists may never know precisely where and when life emerged.272
This statement, by a pioneer of the struggle to find an evolutionary explanation for the origin of life, clearly reflects the despair felt by evolutionist scientists over the cul-de-sac they find themselves in.
So far, we have examined how impossible the accidental formation of life is. Let us again ignore these impossibilities for just a moment. Let us suppose that millions of years ago a cell was formed which had acquired everything necessary for life, and that it duly "came to life." Evolution again collapses at this point. For even if this cell had existed for a while, it would eventually have died and after its death, nothing would have remained, and everything would have reverted to where it had started. This is because this first living cell, lacking any genetic information, would not have been able to reproduce and start a new generation. Life would have ended with its death.
The genetic system does not only consist of DNA. The following things must also exist in the same environment: enzymes to read the code on the DNA, messenger RNA to be produced after reading these codes, a ribosome to which messenger RNA will attach according to this code, transfer RNA to transfer the amino acids to the ribosome for use in production, and extremely complex enzymes to carry out numerous intermediary processes. Such an environment cannot exist anywhere apart from a totally isolated and completely controlled environment such as the cell, where all the essential raw materials and energy resources exist.
As a result, organic matter can self-reproduce only if it exists as a fully developed cell, with all its organelles. This means that the first cell on earth was formed "all of a sudden," together with its extraordinarily complex structure.
So, if a complex structure came into existence all of a sudden, what does this mean?
Let us ask this question with an example. Let us liken the cell to a high-tech car in terms of its complexity. (In fact, the cell is a much more complex and developed system than a car.) Now let us ask the following question: What would you think if you went out hiking in the depths of a thick forest and ran across a brand-new car among the trees? Would you imagine that various elements in the forest had come together by chance over millions of years and produced such a vehicle? All the parts in the car are made of products such as iron, copper, and rubber—the raw ingredients for which are all found on the earth—but would this fact lead you to think that these materials had synthesized "by chance" and then come together and manufactured such a car?
There is no doubt that anyone with a sound mind would realize that the car was the product of an intelligent design, and wonder what it was doing there in the middle of the forest. The sudden emergence of a complex structure in a complete form, quite out of the blue, shows that this is the work of an intelligent design.
Believing that pure chance can produce perfect structures goes well beyond the bounds of reason. Yet every "explanation" put forward by the theory of evolution regarding the origin of life is like that. One outspoken authority on this issue is the famous French zoologist Pierre-Paul Grassé. Grassé is an evolutionist, yet he acknowledges that Darwinist theory is unable to explain life and makes a point about the logic of "coincidence," which is the backbone of Darwinism:
The opportune appearance of mutations permitting animals and plants to meet their needs seems hard to believe. Yet the Darwinian theory is even more demanding: A single plant, a single animal would require thousands and thousands of lucky, appropriate events. Thus, miracles would become the rule: events with an infinitesimal probability could not fail to occur… There is no law against daydreaming, but science must not indulge in it.273
All living things in the world, all of which are clear examples of the intelligent planning we have just been discussing, are at the same time living evidence that coincidence can have no role to play in their existence. Each of its component parts—never mind a whole living creature—contains structures and systems so complex that they cannot be the work of coincidence. We need go no further than our own bodies to find examples of this.
One example of this is our eyes. The human eye sees by the working together of some 40 separate parts. If one of these is not present, the eye will be useless. Each of these 40 parts possesses complex structures within itself. The retina at the back of the eye, for instance, is made up of 11 layers. Each layer has a different function. The chemical processes that go on inside the retina are so complex that they can only be explained with pages full of formulae and diagrams.
The theory of evolution is unable to account for the emergence of even such a flawless and complex structure as a single eye by means of "accident," let alone life itself, or mankind.
So, what do these extraordinary features in living things prove to us about the origin of life? As we made clear in the opening part of this book, only two different accounts can be given regarding the origin of life. One is the fallacious evolutionary explanation, the other the evident "fact of creation." As explained throughout the book, the evolution claim is impossible, and scientific discoveries prove the truth of creation. This truth may surprise some scientists, who from the nineteenth century to the present have seen the concept of "creation" as unscientific, but science can only progress by overcoming shocks of this kind and accepting the truth. Chandra Wickramasinghe describes the reality he faced as a scientist who had been told throughout his life that life had emerged as a result of chance coincidences:
From my earliest training as a scientist, I was very strongly brainwashed to believe that science cannot be consistent with any kind of deliberate creation. That notion has had to be painfully shed. At the moment, I can't find any rational argument to knock down the view which argues for conversion to Allah. We used to have an open mind; now we realize that the only logical answer to life is creation - and not accidental random shuffling.274
238 Michael Denton, Evolution: A Theory in Crisis, Burnett Books, London, 1985, pp. 328, 342.
239 Charles Darwin, Life and Letter of Charles Darwin, vol. II, From Charles Darwin to J. Do Hooker, March 29, 1863
240 W. R. Bird, The Origin of Species Revisited, Thomas Nelson Co., Nashville, 1991, pp. 298-99.
241 "Hoyle on Evolution," Nature, vol. 294, November 12, 1981, p. 105.
242 Fred Hoyle, Chandra Wickramasinghe, Evolution from Space, Simon & Schuster, New York, 1984, p. 130. (emphasis added)
243 Fabbri Britannica Bilim Ansiklopedisi (Fabbri Britannica Science Encyclopaedia), vol. 2, no. 22, p. 519.
244 Alexander I. Oparin, Origin of Life, Dover Publications, New York, 1936, 1953 (reprint), p. 196.
245 Klaus Dose, "The Origin of Life: More Questions Than Answers," Interdisciplinary Science Reviews, vol. 13, no. 4, 1988, p. 348. (emphasis added)
246 Horgan, John, The End of Science, M. A. Addison-Wesley, 1996, p. 138. (emphasis added)
247 Jeffrey Bada, Earth, "Life's Crucible," February 1998, p. 40. (emphasis added)
248 Richard B. Bliss, Gary E. Parker, Duane T. Gish, Origin of Life, C.L.P. Publications, 3rd ed., California, 1990, pp. 14-15.
249 Kevin Mc Kean, Bilim ve Teknik (Science and Technology), no. 189, p. 7.
250 J. P. Ferris, C. T. Chen, "Photochemistry of Methane, Nitrogen, and Water Mixture As a Model for the Atmosphere of the Primitive Earth," Journal of American Chemical Society, vol. 97:11, 1975, p. 2964.
251 "New Evidence on Evolution of Early Atmosphere and Life," Bulletin of the American Meteorological Society, vol. 63, November 1982, pp. 1328-1330.
252 Richard B. Bliss & Gary E. Parker, Duane T. Gish, Origin of Life, C.L.P. Publications, 3rd ed., California, 1990, p. 16.
253 "Life's Crucible," Earth, February 1998, p. 34. (emphasis added)
254 "The Rise of Life on Earth," National Geographic, March 1998, p. 68. (emphasis added)
255 W. R. Bird, The Origin of Species Revisited, Thomas Nelson Co., Nashville, 1991, p. 325.(emphasis added)
256 Richard Dickerson, "Chemical Evolution," Scientific American, vol. 239:3, 1978, p. 75. Chemist Richard Dickerson explains the reason for this in this way: "If polymeric chains of proteins and nucleic acids are to be forged out of their precursor monomers, a molecule of water must be removed at each link in the chain. It is therefore hard to see how polymerization could have proceeded in the aqueous environment of the primitive ocean, since the presence of water favors depolymerization rather than polymerization."
257 S. W. Fox, K. Harada, G. Kramptiz, G. Mueller, "Chemical Origin of Cells," Chemical Engineering News, June 22, 1970, p. 80.
258 Paul Auger, De La Physique Theorique a la Biologie, 1970, p. 118.
259 Francis Crick, Life Itself: It's Origin and Nature, New York, Simon & Schuster, 1981, p. 88. (emphasis added)
260 Ali Demirsoy, Kalitim ve Evrim (Inheritance and Evolution), Meteksan Publishing Co., Ankara, 1984, p. 39.
261 John Horgan, "In the Beginning," Scientific American, vol. 264, February 1991, p. 119. (emphasis added)
262 Homer Jacobson, "Information, Reproduction and the Origin of Life," American Scientist, January 1955, p. 121.
263 Douglas R. Hofstadter, Gödel, Escher, Bach: An Eternal Golden Braid, Vintage Books, New York, 1980, p. 548. (emphasis added)
264 Leslie E. Orgel, "The Origin of Life on Earth," Scientific American, vol. 271, October 1994, p. 78. (emphasis added)
265 Cairns-Smith, Alexander G., "The First Organisms," Scientific American, 252: 90, June 1985. (emphasis added)
266 Michael Denton, Evolution: A Theory in Crisis, London: Burnett Books, 1985, p. 351.
267 John Horgan, "In the Beginning," Scientific American, vol. 264, February 1991, p. 119.
268 G. F. Joyce, L. E. Orgel, "Prospects for Understanding the Origin of the RNA World," In the RNA World, Cold Spring Harbor Laboratory Press, New York, 1993, p. 13.
269 Jacques Monod, Chance and Necessity, New York, 1971, p. 143. (emphasis added)
270 Dover, Gabby L., Looping the Evolutionary loop, review of the origin of life from the birth of life to the origin of language, Nature, 1999, vol. 399, p. 218. (emphasis added)
271 Leslie E. Orgel, "The Origin of Life on the Earth," Scientific American, October 1994, vol. 271, p. 78.
272 Horgan, John, The End of Science, M. A. Addison-Wesley, 1996, p. 139.
273 Pierre-P Grassé, Evolution of Living Organisms, Academic Press, New York, 1977, p. 103. (emphasis added)
274 Chandra Wickramasinghe, Interview in London Daily Express, August 14, 1981.